Abstract
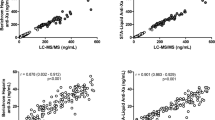
The objectives of this study are to compare steady-state trough (Cmin,ss) and peak (Cmax,ss) concentrations of rivaroxaban between Asians and Caucasians and to evaluate the relationship between rivaroxaban concentrations and prothrombin time/international normalized ratio (PT/INR). Recruited patients were advised on the time to take rivaroxaban. Cmin,ss and PT/INR were taken when patients arrived. Cmax,ss and PT/INR were drawn between 2 and 4 h later after the patient took rivaroxaban with food. Thirty patients were included in the analyses: 57% (n = 17) males and 43% (n = 13) females, 77% (n = 23) on 20 mg and 23% (n = 7) on 15 mg. Median PTtrough and PTpeak are moderately correlated with Cmin,ss (r2 = 0.43) and Cmax,ss (r2 = 0.49), respectively. Patients on 15 mg have lower Cmin,ss and Cmax,ss versus Caucasians [12 ng/ml vs. 57 ng/ml (Cmin,ss); 87 ng/ml vs. 229 ng/ml (Cmax,ss), p < 0.01 for both]. Patients on 20 mg also have lower Cmin,ss and Cmax,ss versus Caucasians [14 ng/ml vs. 44 ng/ml (Cmin,ss); 101 ng/ml vs. 249 ng/ml (Cmax,ss), p < 0.01 for both]. Subgroup analysis shows patients with BMI ≥ 30 have lower Cmax,ss than patients with BMI < 30 [80.47 ng/ml vs. 124 (p = 0.014)]. Cmin,ss and Cmax,ss were lower in Singaporeans than Caucasians. This may have an impact on the effectiveness of rivaroxaban in Singaporeans. Patients with higher BMI may not benefit similarly as patients with lower BMI. Lastly, the Dade Innovin reagent’s measure of PT/INR is not sensitive towards changes in rivaroxaban concentrations.


Similar content being viewed by others
References
Tanigawa T, Kaneko M, Hashizume K et al (2013) Model-based dose selection for phase III rivaroxaban study in Japanese patients with non-valvular atrial fibrillation. Drug Metab Pharmacokinet 28(1):59–70
Hori M, Matsumoto M, Tanahashi N et al (2012) Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J 76(9):2104–2111
Kreutz R (2014) Pharmacokinetics and pharmacodynamics of rivaroxaban–an oral, direct factor Xa inhibitor. Curr Clin Pharmacol 9(1):75–83
Samama MM, Contant G, Spiro TE et al (2013) Laboratory assessment of rivaroxaban: a review. Thromb J 11(1):11
Patel MR, Mahaffey KW, Garg J et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891
Reilly PA, Lehr T, Haertter S et al (2014) The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (randomized evaluation of long-term anticoagulation therapy). J Am Coll Cardiol 63(4):321–328
Eikelboom JW, Connolly SJ, Brueckmann M et al (2013) Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 369(13):1206–1214
Camm AJ, Amarenco P, Haas S et al (2016) XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J 37(14):1145–1153
First Pan-Asian Real-World Study on the use of once daily direct factor Xa inhibitor for stroke prevention in patients with atrial fibrillation In: Bayer: science for a better life global news. http://bayer.com/en/posts/detail/19/258. Accessed 17 Jan 2018
Streiff MB, Agnelli G, Connors JM et al (2016) Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis 41(1):32–67
Mueck W, Stampfuss J, Kubitza D, Becka M (2014) Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 53(1):1–16
Ahmed S, Zhou Z, Zhou J, Chen SQ (2016) Pharmacogenomics of drug metabolizing enzymes and transporters: relevance to precision medicine. Genom Proteom Bioinform 14(5):298–313
Ozen F, Silan C, Uludag A et al (2011) Association between ABCB1 (MDR1) gene 3435 C> T polymorphism and colchicine unresponsiveness of FMF patients. Ren Fail 33(9):899–903
Zhao X, Sun P, Zhou Y et al (2009) Safety, pharmacokinetics and pharmacodynamics of single/multiple doses of the oral, direct Factor Xa inhibitor rivaroxaban in healthy Chinese subjects. Br J Clin Pharmacol 68(1):77–88
Van dyk M, Marshall JC, Sorich MJ, Wood LS, Rowland A (2018) Assessment of inter-racial variability in CYP3A4 activity and inducibility among healthy adult males of Caucasian and South Asian ancestries. Eur J Clin Pharmacol 74(7):913–920
Acknowledgements
Dr. Wang Jiexun for her help with the statistics. Dr. Ong Hean Yee, Dr. Lee Chee Wan, Dr. Ling Lee Fong, Dr. Leow Khang Leng, Dr. Dinna Soon Kar Nee, Dr. Michael Liang Mao Chen, Dr. Syed Saqib Imran, Dr. Justin Tang I-Shing, Dr. Cliff Wong Chun Pong, Dr. Ramkumar Sekar for letting us recruit your patients. Ms. Soh Lay See for extending a hand to help us with the logistics of the study.
Funding
This study was funded by National Medical Research Council (NRMC) Centre Grant Pitch-For-Fund. Award No: PFF15001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
This study was approved by the Domain Specific Review Board (DSRB) prior to the commencement of the study (DSRB approval reference number: 2016/00195).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ng Tsai, H.O., Goh, J.J.N., Aw, J.W.X. et al. Comparison of rivaroxaban concentrations between Asians and Caucasians and their correlation with PT/INR. J Thromb Thrombolysis 46, 541–548 (2018). https://doi.org/10.1007/s11239-018-1726-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1726-y