Abstract
Purpose
Overactive bladder (OAB) is a symptom-based disease; therefore, clinical trials to evaluate treatments for OAB employ a range of efficacy endpoints. Since factors that influence efficacy endpoints can affect trial outcomes, their identification could aid in the design of future OAB clinical trials. We investigated factors influencing different efficacy endpoints used in clinical trials with OAB patients and examined their characteristics to determine future clinical trial strategies for new medicinal treatments for OAB.
Methods
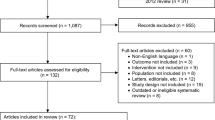
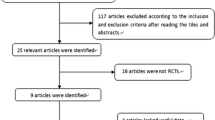
Data from placebo-controlled double-blind trials in patients with OAB were extracted via a systematic literature review. The integrated differences for efficacy endpoints were calculated. Heterogeneity was assessed using the Q statistic and I2 statistic. Factors influencing efficacy endpoints were identified through univariate and multivariate meta-regression analyses.
Results
Forty-one controlled trials were analyzed. Substantial heterogeneity between studies was observed for each efficacy endpoint (P > 0.001, I2 > 70%). We found with multivariate meta-regression analysis that period of recording in a bladder diary and year of publication were significantly likely to influence the change from baseline in the mean number of urgency episodes in 24 h, year of publication and gender were significantly likely to influence the change from baseline in the mean number of micturitions in 24 h, and gender was significantly likely to influence the change from baseline in the mean volume voided per micturition. In contrast, there were no factors significantly associated with change from baseline in the mean number of incontinence episodes in 24 h.
Conclusions
We identified that change from baseline in the mean number of incontinence episodes in 24 h should serve as a relatively stable endpoint. In contrast, we identified factors influencing other endpoints, and the identified factors should be taken into account when planning and conducting future clinical trials.

Similar content being viewed by others
References
Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ (2001) How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87(9):760–766
Homma Y, Yamaguchi O, Hayashi K (2005) An epidemiological survey of overactive bladder symptoms in Japan. BJU Int 96(9):1314–1318
Basra RK, Wagg A, Chapple C et al (2008) A review of adherence to drug therapy in patients with overactive bladder. BJU Int 102(7):774–779
Buser N, Ivic S, Kessler TM, Kessels AG, Bachmann LM (2012) Efficacy and adverse events of antimuscarinics for treating overactive bladder: network meta-analyses. Eur Urol 62(6):1040–1060
Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D (2008) The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54(3):543–562
Sears CL, Lewis C, Noel K, Albright TS, Fischer JR (2010) Overactive bladder medication adherence when medication is free to patients. J Urol 183(3):1077–1081
Wagg A, Compion G, Fahey A, Siddiqui E (2012) Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 110(11):1767–1774
Haylen BT, Dde Ridder, Freeman RM et al (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 29(1):4–20
EMA (2013) Guideline on the clinical investigation of medicinal products for the treatment of urinary incontinence. CPMP/EWP/18/01/Rev.1. London. (cited 2015 June 13) URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500146177.pdf
PFSB (2006) Guidelines for clinical evaluation of drug for overactive bladder or incontinence. Notification No. 0628001 of the Evaluation and Licensing Division. Tokyo. (cited 2015 June 13) https://www.pmda.go.jp/files/000206216.pdf
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 89(9):873–880
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 17(1):1–12
R Foundation for Statistical Computing. R: a language and environment for statistical computing. https://www.R-project.org/. Accessed 1 Feb 2018
Chapple CR, Dvorak V, Radziszewski P et al (2013) A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J 24(9):1447–1458
Chapple CR, Abrams P, Andersson KE et al (2014) Phase II study on the efficacy and safety of the EP1 receptor antagonist ONO-8539 for nonneurogenic overactive bladder syndrome. J Urol 191(1):253–260
Chapple CR, Amarenco G, Lopez Aramburu MA et al (2013) A proof-of-concept study: mirabegron, a new therapy for overactive bladder. Neurourol Urodyn 32(8):1116–1122
Herschorn S, Barkin J, Castro-Diaz D et al (2013) A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the beta(3) adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology 82(2):313–320
Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S (2013) Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 189(4):1388–1395
Khullar V, Amarenco G, Angulo JC et al (2013) Efficacy and tolerability of mirabegron, a beta(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European–Australian phase 3 trial. Eur Urol 63(2):283–295
Ohlstein EH, von Keitz A, Michel MC (2012) A multicenter, double-blind, randomized, placebo-controlled trial of the beta3-adrenoceptor agonist solabegron for overactive bladder. Eur Urol 62(5):834–840
Digesu GA, Verdi E, Cardozo L, Olivieri L, Khullar V, Colli E (2012) Phase IIb, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to determine effects of elocalcitol in women with overactive bladder and idiopathic detrusor overactivity. Urology 80(1):48–54
Yamaguchi O, Nishizawa O, Takeda M et al (2011) Efficacy, safety and tolerability of fesoterodine in asian patients with overactive bladder. Low Urin Tract Symptoms 3(1):43–50
Gotoh M, Yokoyama O, Nishizawa O (2011) Propiverine hydrochloride in Japanese patients with overactive bladder: a randomized, double-blind, placebo-controlled trial. Int J Urol 18(5):365–373
Lee KS, Lee HW, Choo MS et al (2010) Urinary urgency outcomes after propiverine treatment for an overactive bladder: the ‘Propiverine study on overactive bladder including urgency data’. BJU Int 105(11):1565–1570
Zat’ura F, Vsetica J, Abadias M et al (2010) Cizolirtine citrate is safe and effective for treating urinary incontinence secondary to overactive bladder: a phase 2 proof-of-concept study. Eur Urol 57(1):145–152
Chu F, Smith N, Uchida T (2009) Efficacy and safety of solifenacin succinate 10 mg once daily: a multicenter, phase III, randomized, double-blind, placebo-controlled, parallel-group trial in patients with overactive bladder. Curr Ther Res Clin Exp 70(6):405–420
Homma Y, Yamaguchi O (2009) A randomized, double-blind, placebo- and propiverine-controlled trial of the novel antimuscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol 16(5):499–506
Homma Y, Yamaguchi T, Yamaguchi O (2008) A randomized, double-blind, placebo-controlled phase II dose-finding study of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol 15(9):809–815
Martinez-Garcia R, Abadias M, Arano P et al (2009) Cizolirtine citrate, an effective treatment for symptomatic patients with urinary incontinence secondary to overactive bladder: a pilot dose-finding study. Eur Urol 56(1):184–190
Nitti VW, Dmochowski R, Sand PK et al (2007) Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol 178(6):2488–2494
Chapple C, Van Kerrebroeck P, Tubaro A et al (2007) Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol 52(4):1204–1212
Dmochowski RR, Sand PK, Zinner NR, Staskin DR (2008) Trospium 60 mg once daily (QD) for overactive bladder syndrome: results from a placebo-controlled interventional study. Urology 71(3):449–454
Staskin D, Sand P, Zinner N, Dmochowski R (2007) Once daily trospium chloride is effective and well tolerated for the treatment of overactive bladder: results from a multicenter phase III trial. J Urol 178(3 Pt 1):978–983 (discussion 83–84)
Yamaguchi O, Marui E, Kakizaki H et al (2007) Randomized, double-blind, placebo- and propiverine-controlled trial of the once-daily antimuscarinic agent solifenacin in Japanese patients with overactive bladder. BJU Int 100(3):579–587
Chapple CR, Patroneva A, Raines SR (2006) Effect of an ATP-sensitive potassium channel opener in subjects with overactive bladder: a randomized, double-blind, placebo-controlled study (ZD0947IL/0004). Eur Urol 49(5):879–886
Cardozo L, Lisec M, Millard R et al (2004) Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol 172(5 Pt 1):1919–1924
Chapple CR, Rechberger T, Al-Shukri S et al (2004) Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int 93(3):303–310
Jacquetin B, Wyndaele J (2001) Tolterodine reduces the number of urge incontinence episodes in patients with an overactive bladder. Eur J Obstet Gynecol Reprod Biol 98(1):97–102
Malone-Lee JG, Walsh JB, Maugourd MF (2001) Tolterodine: a safe and effective treatment for older patients with overactive bladder. J Am Geriatr Soc 49(6):700–705
Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A (2001) Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology 57(3):414–421
Yamaguchi O, Marui E, Igawa Y et al (2015) Efficacy and safety of the selective beta3-adrenoceptor agonist mirabegron in Japanese patients with overactive bladder: a randomized, double-blind, placebo-controlled, dose-finding study. Low Urin Tract Symptoms 7(2):84–92
Song M, Kim JH, Lee KS et al (2015) The efficacy and tolerability of tarafenacin, a new muscarinic acetylcholine receptor M3 antagonist in patients with overactive bladder; randomised, double-blind, placebo-controlled phase 2 study. Int J Clin Pract 69(2):242–250
Yamaguchi O, Marui E, Kakizaki H et al (2014) Phase III, randomised, double-blind, placebo-controlled study of the beta3-adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder. BJU Int 113(6):951–960
Betschart C, von Mandach U, Seifert B et al (2013) Randomized, double-blind placebo-controlled trial with Bryophyllum pinnatum versus placebo for the treatment of overactive bladder in postmenopausal women. Phytomedicine 20(3–4):351–358
Drutz HP, Appell RA, Gleason D, Klimberg I, Radomski S (1999) Clinical efficacy and safety of tolterodine compared to oxybutynin and placebo in patients with overactive bladder. Int Urogynecol J Pelvic Floor Dysfunct 10(5):283–289
Van Kerrebroeck PE, Amarenco G, Thuroff JW et al (1998) Dose-ranging study of tolterodine in patients with detrusor hyperreflexia. Neurourol Urodyn 17(5):499–512
Herschorn S, Heesakkers J, Castro-Diaz D, Wang JT, Brodsky M, Guan Z (2008) Effects of tolterodine extended release on patient perception of bladder condition and overactive bladder symptoms*. Curr Med Res Opin 24(12):3513–3521
Chancellor M, Freedman S, Mitcheson HD, Antoci J, Primus G, Wein A (2000) Tolterodine, an effective and well tolerated treatment for urge incontinence and other overactive bladder symptoms. Clin Drug Investig 19(2):83–91
Yamaguchi O, Marui E, Kakizaki H et al (2006) Solifenacin succinate phase II clinical trial—dose-finding study. Jpn Pharmacol Ther 2006(34):S47–S67
Rogers R, Bachmann G, Jumadilova Z et al (2008) Efficacy of tolterodine on overactive bladder symptoms and sexual and emotional quality of life in sexually active women. Int Urogynecol J Pelvic Floor Dysfunct 19(11):1551–1557
Green SA, Alon A, Ianus J, McNaughton KS, Tozzi CA, Reiss TF (2006) Efficacy and safety of a neurokinin-1 receptor antagonist in postmenopausal women with overactive bladder with urge urinary incontinence. J Urol 176(6 Pt 1):2535–2540 (discussion 40)
Bayer (2000) A study to assess if 10 mg Vardenafil (BAY38-9456) taken twice daily for 6 weeks has an effect on bladder function. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2015 Jun 13]. https://clinicaltrials.gov/ct2/show/NCT00478881?term=NCT00478881&rank=1. NLM Identifier: NCT00478881
Sunovion, Dainippon Sumitomo Pharma America, ICON Clinical Research, ClinPhone ICP (2000) SMP-986 phase 2 proof of concept in patients with overactive bladder syndrome (OABS). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [2015 Jun 13]. https://clinicaltrials.gov/ct2/show/NCT00409539?term=NCT00409539&rank=1. NLM Identifier: NCT00409539
Astellas Pharma Inc. (2000) Study of 2 doses of solifenacin succinate in female subjects with overactive bladder. (SHRINK). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [2015 Jun 13]. https://clinicaltrials.gov/ct2/show/NCT01093534?term=NCT01093534&rank=1. NLM Identifier: NCT01093534
Homma Y, Ando T, Yoshida M et al (2002) Voiding and incontinence frequencies: variability of diary data and required diary length. Neurourol Urodyn 21(3):204–209
Schick E, Jolivet-Tremblay M, Dupont C et al (2003) Frequency–volume chart: the minimum number of days required to obtain reliable results. Neurourol Urodyn 22(2):92–96
Brown JS, McNaughton KS, Wyman JF et al (2003) Measurement characteristics of a voiding diary for use by men and women with overactive bladder. Urology 61(4):802–809
Ku JH, Jeong IG, Lim DJ et al (2004) Voiding diary for the evaluation of urinary incontinence and lower urinary tract symptoms: prospective assessment of patient compliance and burden. Neurourol Urodyn 23(4):331–335
Abrams P, Donovan JL, de la Rosette JJMCH et al (1997) International Continence Society “Benign Prostatic Hyperplasia” Study: background, aims, and methodology. Neurourol Urodyn 16(2):79–91
Nielsen KK, Nording J, Hald T (1994) Critical review of the diagnosis of prostatic obstruction. Neurourol Urodyn 13(3):201–217
Lee S, Malhotra B, Creanga D et al (2009) A meta-analysis of the placebo response in antimuscarinic drug trials for overactive bladder. BMC Med Res Methodol 22:9–55
Acknowledgements
Editorial support was provided by Kentarou Kuroishi from Astellas Pharma, Inc., and Andrew Lewis from Astellas Pharma Global Development, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest that are directly relevant to this research. Shingo Iino is an employee of Astellas Pharma, Inc.
Rights and permissions
About this article
Cite this article
Iino, S., Kaneko, M. & Narukawa, M. Factors influencing efficacy endpoints in clinical trials for new oral medicinal treatments for overactive bladder: a systematic literature review and meta-analysis. Int Urol Nephrol 50, 1021–1030 (2018). https://doi.org/10.1007/s11255-018-1869-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1869-y