Abstract
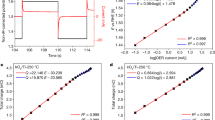
Although hydrazine (N2H4) oxidation in an electrochemical environment has been of great interest for years, its intrinsic electron transfer kinetics remain uncertain. We report that the phenomenological Butler-Volmer (BV) theory is not appropriate for interpreting the process of hydrazine oxidation for which an astonishingly wide range of transfer coefficients, Tafel slopes and diffusion coefficient have been previously reported. Rather Tafel analysis for voltammetry recorded at Glassy Carbon (GC) electrodes reveals a strong potential dependence of the anodic transfer coefficient, consistent with the symmetric Marcus-Hush (sMH) theory. According to the relationship \(\beta = {{\lambda + FE_f^0} \over {2\lambda }} - {F \over {2\lambda }}E\), the reorganization energy (0.35±0.07 eV) and an approximate formal potential of the rate-determining first electron transfer were successfully extracted from the voltammetric responses.

Similar content being viewed by others
References
Furst A, Berlo RC, Hooton S. Chem Rev, 1965, 65: 51–68
Lu Z, Sun M, Xu T, Li Y, Xu W, Chang Z, Ding Y, Sun X, Jiang L. Adv Mater, 2015, 27: 2361–2366
Wang T, Wang Q, Wang Y, Da Y, Zhou W, Shao Y, Li D, Zhan S, Yuan J, Wang H. Angew Chem Int Ed, 2019, 58: 13466–13471
Feng G, Kuang Y, Li P, Han N, Sun M, Zhang G, Sun X. Adv Sci, 2017, 4: 1600179
Wang B, Cao X. Electroanalysis, 1992, 4: 719–724
Baron R, Šljukić B, Salter C, Crossley A, Compton R. Electroanalysis, 2007, 19: 1062–1068
Xiong L, Compton RG. Int J Electrochem Sci, 2014, 9: 7152–7181
Bruice TC, Bruno JJ, Chou WS. J Am Chem Soc, 1963, 85: 1659–1669
Moreno JH, Diamond JM. Nature, 1974, 247: 368–369
Hayon E, Simic M. J Am Chem Soc, 1972, 94: 42–47
Korovin NV, Yanchuk BN. Electrochim Acta, 1970, 15: 569–580
Rosca V, Duca M, de Groot MT, Koper MTM. Chem Rev, 2009, 109: 2209–2244
Kocak CC, Altin A, Aslisen B, Kocak S. Int J Electrochem Sci, 2016, 11: 233–249
Maduraiveeran G, Ramaraj R. J Anal Sci Technol, 2017, 8: 1
Koçak S, Aslışen B. Sens Actuat B-Chem, 2014, 196: 610–618
Batchelor-McAuley C, Kätelhön E, Barnes EO, Compton RG, Laborda E, Molina A. ChemistryOpen, 2015, 4: 224–260
Chidsey CED. Science, 1991, 251: 919–922
Laborda E, Henstridge MC, Batchelor-McAuley C, Compton RG. Chem Soc Rev, 2013, 42: 4894–4905
Feldberg SW. Anal Chem, 2010, 82: 5176–5183
Ding Z, Quinn BM, Bard AJ. J Phys Chem B, 2001, 105: 6367–6374
Wang Y, Laborda E, Henstridge MC, Martinez-Ortiz F, Molina A, Compton RG. J Electroanal Chem, 2012, 668: 7–12
Laborda E, Henstridge MC, Compton RG. J Electroanal Chem, 2012, 667: 48–53
Butler JAV. Trans Faraday Soc, 1932, 28: 379
Erdey-Grúz T, Volmer M. Z für Physikalische Chem, 1930, 150A: 203
Bordwell FG, Boyle WJ. J Am Chem Soc, 1972, 94: 3907–3911
Formosinho SJ. J Chem Soc Perkin Trans 2, 1987, 61
Marcus RA. J Chem Phys, 1956, 24: 966–978
Eberson L, Gonzalez-Luque R, Lorentzon J, Merchan M, Roos BO. J Am Chem Soc, 1993, 115: 2898–2902
Hush NS. J Chem Phys, 1958, 28: 962–972
Hush NS. J Electroanal Chem, 1999, 460: 5–29
Appleby AJ, Zagal JH. J Solid State Electrochem, 2011, 15: 1811–1832
Bai P, Bazant MZ. Nat Commun, 2014, 5: 3585
Laborda E, Henstridge MC, Compton RG. J Electroanal Chem, 2012, 681: 96–102
Savéant J-M. Elements of molecular and biomolecular electrochemistry. New Jersey: Willey-VCH, 2006.
Forster RJ, Faulkner LR. J Am Chem Soc, 1994, 116: 5444–5452
Madhiri N, Finklea HO. Langmuir, 2006, 22: 10643–10651
Kozub BR, Henstridge MC, Batchelor-McAuley C, Compton RG. ChemPhysChem, 2011, 12: 2806–2815
Henstridge MC, Wang Y, Limon-Petersen JG, Laborda E, Compton RG. Chem Phys Lett, 2011, 517: 29–35
Laborda E, Wang Y, Henstridge MC, Martínez-Ortiz F, Molina A, Compton RG. Chem Phys Lett, 2011, 512: 133–137
Henstridge MC, Laborda E, Dickinson EJF, Compton RG. J Electroanal Chem, 2012, 664: 73–79
Suwatchara D, Rees NV, Henstridge MC, Laborda E, Compton RG. J Electroanal Chem, 2012, 665: 38–44
Zeng Y, Bai P, Smith RB, Bazant MZ. J Electroanal Chem, 2015, 748: 52–57
Rudolph M, Reddy DP, Feldberg SW. Anal Chem, 1994, 66: 589A–600A
Compton RG, Banks CE. Understanding voltammetry. 3rd Edition. London: World Scientific, 2018.
Elgrishi N, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, Dempsey JL. J Chem Educ, 2018, 95: 197–206
Wang Y, Wan Y, Zhang D. Electrochem Commun, 2010, 12: 187–190
Li D, Lin C, Batchelor-McAuley C, Chen L, Compton RG. J Electroanal Chem, 2018, 826: 117–124
Miao R, Chen L, Shao L, Zhang B, Compton RG. Angew Chem Int Ed, 2019, 58: 12549–12552
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Rights and permissions
About this article
Cite this article
Miao, R., Chen, L. & Compton, R.G. Electro-oxidation of hydrazine shows marcusian electron transfer kinetics. Sci. China Chem. 64, 322–329 (2021). https://doi.org/10.1007/s11426-020-9889-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-020-9889-1