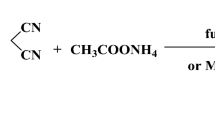Abstract
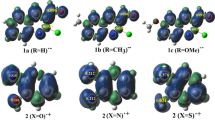
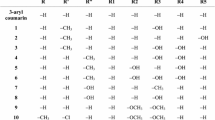
Chalcones are bioactive compounds obtained from either natural sources or synthetic procedures and widely used due to their several biological properties. The most common experimental methodology in obtaining these compounds is Claisen–Schmidt reaction, which is a particular type of aldolic condensation. In this work, we have synthesized 23 chalcones and by density functional theory (DFT) calculation, we have studied the difference in reactivity of the several benzaldehydes and their effects on the yield of this reaction. From molecular orbital descriptors were obtained two quantitative structure–reactivity relationship (QSRR) models based on Hansch’s analysis. The results of this study showed that, for the most benzaldehydes (15 of 23 compounds), their reactivity was correlated with LUMO energy and global Electrophilicity Index (ω) values, which are determined in the first step of Claisen–Schmidt condensation mechanism (nucleophilic addition). Likewise, for the smallest group of benzaldehydes, their reactivity was related to their HOMO and ΔL − H (LUMO − HOMO) energies, which were determined in the second step of the mechanism (trans-elimination). This is the first report of a QSRR model analyzing the yield of chalcone synthesis based on DFT methodology.






Similar content being viewed by others
References
Akansksha AK (2007) Zirconium chloride catalyzed efficient synthesis of 1,3-diaryl-2-propenones in solvent free conditions via aldol condensation. J Mol Catal A Chem 274:212–216. doi:10.1016/j.molcata.2007.05.016
Alegaon SG, Alagawadi KR, Vinod D, Unger B, Khatib NA (2014) Synthesis, pharmacophore modeling, and cytotoxic activity of 2-thioxothiazolidin-4-one derivatives. Med Chem Res 23:5160–5173. doi:10.1007/s00044-014-1087-9
Anto RJ, Kutaan G, Kuttan R, Sathyanarayana K, Rao MNA (1994) Tumor-reducing and antioxidant activities of sydnone-substituted chalcones. J Clin Biochem Nutr 17:73–80. doi:10.3164/jcbn.17.73
Bhagat S, Sharma R, Sawant D, Sharma L, Chakraborti A (2006) LiOH· H2O as a novel dual activation catalyst for highly efficient and easy synthesis of 1, 3-diaryl-2-propenones by Claisen–Schmidt condensation under mild conditions. J Mol Catal A Chem 244:20–24. doi:10.1016/j.molcata.2005.08.039
Carloni P, Alber F, Mannhold R, Kubinyi H, Folkers G (2006) Quantum medicinal chemistry, vol 17. Wiley-VCH, Alemania
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comp Chem 20:129–154. doi:10.1002/(Sici)1096-987x(19990115)20:1<129:Aid-Jcc13>3.0.Co;2-A
De Vleeschouwer F, Van Speybroeck V, Waroquier M, Geerlings P, De Proft F (2007) Electrophilicity and nucleophilicity index for radicals. Org Lett 9:2721–2724. doi:10.1021/ol071038k
Duchowicz PR, Castro EA (2009) QSPR studies on aqueous solubilities of drug-like compounds. Int J Mol Sci 10:2558–2577. doi:10.3390/ijms10062558
Ege S (2000) Reacciones Concertadas Química Orgánica Estructura y Reactividad, vol TOMO 1. Editorial Revertè, España, pp 1297–1356
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford
Golbraikh A, Alexander T (2002) Beware of q2! J Mol Graph Model 20:269. doi:10.1016/S1093-3263(01)00123-1
Jeon JH, Kim SJ, Kim CG, Kim JK, Jun JG (2012) Synthesis of biologically active chalcones and their anti-inflammatory effects. Bull Korean Chem Soc 33:953–957. doi:10.5012/bkcs.2012.33.3.953
Kamal A, Prabhakar S, Janaki Ramaiah M, Venkat Reddy P, Ratna Reddy C, Mallareddy A, Shankaraiah N, Lakshmi Narayan Reddy T, Pushpavalli SN, Pal-Bhadra M (2011) Synthesis and anticancer activity of chalcone-pyrrolobenzodiazepine conjugates linked via 1,2,3-triazole ring side-armed with alkane spacers. Eur J Med Chem 46:3820–3831. doi:10.1016/j.ejmech.2011.05.050
Karelson M, Lobanov VS (1996) Quantum-chemical descriptor in QSAR/QSPR studies. Chem Rev 96:1027–1043. doi:10.1021/cr950202r
Li W, Xu K, Xu L, Hu J, Ma F, Guo Y (2010) Preparation of highly ordered mesoporous AlSBA-15–SO3H hybrid material for the catalytic synthesis of chalcone under solvent-free condition. Appl Surf Sci 256:3183–3190
Liu Z, Lee W, Kim SN, Yoon G, Cheon SH (2011) Design, synthesis, and evaluation of bromo-retrochalcone derivatives as protein tyrosine phosphatase 1B inhibitors. Bioorg Med Chem Lett 21:3755–3758. doi:10.1016/j.bmcl.2011.04.057
Lopez JM, Ensuncho AE, Robles JR (2013) Global and local reactivity descriptors for the design of new anticancer drugs based on cis-platinum(II). Quim Nova 36:1308–1317. doi:10.1590/S0100-40422013000900006
Luo Y, Song R, Li Y, Zhang S, Liu ZJ, Fu J, Zhu HL (2012) Design, synthesis, and biological evaluation of chalcone oxime derivatives as potential immunosuppressive agents. Bioorg Med Chem Lett 22:3039–3043. doi:10.1016/j.bmcl.2012.03.080
Moens J, Geerlings P, Roos G (2007) A conceptual DFT approach for the evaluation and interpretation of redox potentials. Chem Eur J 13:8174–8184. doi:10.1002/chem.200601896
Narender T, Reddy KP (2007) A simple and highly efficient method for the synthesis of chalcones by using borontrifluoride-etherate. Tetrahedron Lett 48:3177–3180. doi:10.1016/j.tetlet.2007.03.054
Narender T, Venkateswarlu K, Nayak BV, Sakar S (2011) A new chemical access for 3′-acetyl-4′-hydroxychalcones using borontrifluoride–etherate via a regioselective Claisen–Schmidt condensation and its application in the synthesis of chalcone hybrids. Tetrahedron Lett 52:5794–5798. doi:10.1016/j.tetlet.2011.08.120
Parr RG, Von Szentpaly L, Liu SB (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. doi:10.1021/Ja983494x
Patil AB, Bhanage BM (2013) Novel and green approach for the nanocrystalline magnesium oxide synthesis and its catalytic performance in Claisen–Schmidt condensation. Catal Commun 36:79–83. doi:10.1016/j.catcom.2013.03.012
Pearson RG (1993) The principle of maximum hardness. Acc Chem Res 26:250–255. doi:10.1021/Ar00029a004
Petrov O, Ivanova Y, Gerova M (2008) SOCl2/EtOH: catalytic system for synthesis of chalcones. Catal Commun 9:315–316. doi:10.1016/j.catcom.2007.06.013
Pilatova M, Varinska L, Perjesi P, Sarissky M, Mirossay L, Solar P, Ostro A, Mojzis J (2010) In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicol In Vitro 24:1347–1355. doi:10.1016/j.tiv.2010.04.013
Silverstein RM, Webster FX, Kiemle DJ (2005) Spectrometric identification of organic compounds, Seventh edn. Wiley, USA
Sivakumar PM, Prabhakar PK, Doble M (2001) Synthesis, antioxidant evaluation, and quantitative structure–activity relationship studies of chalcones. Med Chem Res 20:482–492. doi:10.1007/s00044-010-9342-1
Sugamoto K, Matsusita YI, Matsui K, Kurogi C, Matsui T (2011) Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 67:5346–5359. doi:10.1016/j.tet.2011.04.104
Yee LC, Wei YC (2012) Statistical modelling of molecular descriptors in QSAR/QSPR. In: Dehmer M, Varmuza K, Bonchev D (eds) Statistical modelling of molecular descriptors in QSAR/QSPR, vol 2. Wiley, Weinheim
Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z (2017) Chalcone: a privileged structure in medicinal chemistry. Chem Rev 117:7762–7810. doi:10.1021/acs.chemrev.7b00020
Acknowledgements
The authors thank the Dirección de Investigación y Postgrado (DGIP) of Universidad Técnica Federico Santa María; scientific initiation project 2015 (PIIC-MM), CONICYT Programa Formación de Capital Humano Avanzado 21130456; Proyecto VRIEA-PUCV “37.0/2017” and Dr. Guillermo Díaz Felming of Laboratorio de Espectroscopia Atómica y Molecular of Universidad de Playa Ancha.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mellado, M., Madrid, A., Martínez, Ú. et al. Hansch’s analysis application to chalcone synthesis by Claisen–Schmidt reaction based in DFT methodology. Chem. Pap. 72, 703–709 (2018). https://doi.org/10.1007/s11696-017-0316-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0316-3