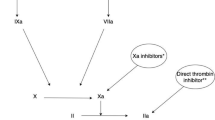
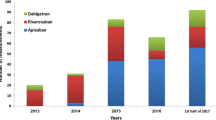
Abstract
Direct oral anticoagulants (DOAC) possess high bioavailability, and their anticoagulant effect is more predictable than that of vitamin K antagonists, hence they do not require routine dose adjustment based on laboratory testing. However, there are circumstances when laboratory testing may be useful, including patients who need to undergo surgery or invasive procedures. Most guidelines state that patients on DOAC may safely undergo surgery/invasive procedures by stopping anticoagulation for a few days before intervention without testing if renal function is within normal limits. This review article discusses the pros and cons of measuring (or not measuring) DOAC levels before surgery/invasive procedures by a multidisciplinary team of experts with different background, including the thrombosis laboratory, clinical thrombosis, internal medicine, cardiology and nephrology. The conclusion is that measuring DOAC with dedicated tests before surgical or invasive procedures is important for patient safety. It provides the best and most direct evidence to rule in (or to rule out) clinically relevant concentrations of residual drugs. Regulatory agencies should urgently approve their use in clinical practice. Hospital administrators should make them available, and clinical laboratories should set up the relative methods and make them available to clinicians.


Similar content being viewed by others
References
Lee LH (2016) DOACs—advances and limitations in real world. Thromb J 14(Suppl 1):17
Alikhan R, Rayment R, Keeling D, Baglin T, Benson G, Green L, Marshall S, Patel R, Pavord S, Rose P, Tait C (2014) The acute management of haemorrhage, surgery and overdose in patients receiving dabigatran. Emerg Med J 31:163–168
Ferrandis R, Castillo J, de Andres J, Gomar C, Gomez-Luque A, Hidalgo F, Llau JV, Sierra P, Torres LM (2013) The perioperative management of new direct oral anticoagulants: a question without answers. Thromb Haemost 110:515–522
Tran H, Joseph J, Young L, McRae S, Curnow J, Nandurkar H, Wood P, McLintock C (2014) New oral anticoagulants: a practical guide on prescription, laboratory testing and peri-procedural/bleeding management. Australian Society on Thrombosis and Haemostasis. Intern Med J 44:525–536
Daniels PR (2015) Peri-procedural management of patients taking oral anticoagulants. BMJ 351:h2391
Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R (2012) Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th edn. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e326S–e350S
Pernod G, Albaladejo P, Godier A, Samama CM, Susen S, Gruel Y, Blais N, Fontana P, Cohen A, Llau JV, Rosencher N, Schved JF, de Maistre E, Samama MM, Mismetti P, Sié P, Working Group on Perioperative Haemostasis (2013) Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor Xa inhibitors: proposals of the Working Group on Perioperative Haemostasis (GIHP). Arch Cardiovasc Dis 106:382–393
Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P, ESC Scientific Document Group (2017) Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 38:2137–2149
Prisco D, Ageno W, Becattini C, D’Angelo A, Davì G, De Cristofaro R, Dentali F, Di Minno G, Falanga A, Gussoni G, Masotti L, Palareti G, Pignatelli P, Santi RM, Santilli F, Silingardi M, Tufano A, Violi F, SIMI (Italian Society of Internal Medicine); FADOI (Federation of Associations of Hospital Doctors on Internal Medicine); SISET (Italian Society for the Study of Haemostasis and Thrombosis) (2017) Italian intersociety consensus on DOAC use in internal medicine. Intern Emerg Med 12:387–406
European Medicine Agency (2017) Annex I, dabigatran summary of product characteristics. http://www.ema.europa.eu/. Accessed Nov 2017
European Medicine Agency (2017) Annex I, rivaroxaban summary of product characteristics. http://www.ema.europa.eu/. Accessed Nov 2017
European Medicine Agency (2017) Annex I, apixaban summary of product characteristics. http://www.ema.europa.eu/. Accessed Nov 2017
European Medicine Agency (2017) Annex I, edoxaban summary of product characteristics. http://www.ema.europa.eu/. Accessed Nov 2017
Tripodi A (2013) The laboratory and the direct oral anticoagulants. Blood 121:4032–4035
Baglin T, Keeling D, Kitchen S, British Committee for Standards in Haematology (2012) Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 159:427–429
Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W (2013) Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 11:756–760
Kitchen S, Gray E, Mackie I, Baglin T, Makris M, BCSH Committee (2014) Measurement of non-coumarin anticoagulants and their effects on tests of haemostasis: guidance from the British Committee for Standards in Haematology. Br J Haematol 166:830–841
Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, Ansell J, American College of Chest Physicians (2008) The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 133(6 Suppl):299S–339S
Pengo V, Cucchini U, Denas G, Erba N, Guazzaloca G, La Rosa L, De Micheli V, Testa S, Frontoni R, Prisco D, Nante G, Iliceto S, Italian Federation of Centers for the Diagnosis of Thrombosis and Management of Antithrombotic Therapies (FCSA) (2009) Standardized low-molecular-weight heparin bridging regimen in outpatients on oral anticoagulants undergoing invasive procedure or surgery: an inception cohort management study. Circulation 119:2920–2927
Schulman S, Carrier M, Lee AY, Shivakumar S, Blostein M, Spencer FA, Solymoss S, Barty R, Wang G, Heddle N, Douketis JD (2015) Perioperative management of dabigatran: a prospective cohort study. Circulation 132:167–173
Douketis JD, Wang G, Chan N, Eikelboom JW, Syed S, Barty R, Moffat KA, Spencer FA, Blostein M, Schulman S (2016) Effect of standardized perioperative dabigatran interruption on the residual anticoagulation effect at the time of surgery or procedure. J Thromb Haemost 14:89–97
Douketis JD, Spyropoulos AC, Anderson JM, Arnold DM, Bates SM, Blostein M, Carrier M, Caprini JA, Clark NP, Coppens M, Dentali F, Duncan J, Gross PL, Kassis J, Kowalski S, Lee AY, Le Gal G, Le Templier G, Li N, MacKay E, Shah V, Shivakumar S, Solymoss S, Spencer FA, Syed S, Tafur AJ, Vanassche T, Thiele T, Wu C, Yeo E, Schulman S (2017) The Perioperative Anticoagulant Use for Surgery Evaluation (PAUSE) Study for patients on a direct oral anticoagulant who need an elective surgery or procedure: design and rationale. Thromb Haemost 117:2415–2424
Tripodi A (2016) To measure or not to measure direct oral anticoagulants before surgery or invasive procedures. J Thromb Haemost 14:1325–1327
Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD (2016) To measure or not to measure direct oral anticoagulants before surgery or invasive procedures: comment. J Thromb Haemost 14:2556–2559
Tripodi A (2016) To measure or not to measure direct oral anticoagulants before surgery or invasive procedures: reply. J Thromb Haemost 14:2559–2561
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Gr(oup: KDIGO 2012 (2013) Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:19–62
Michel WM, Grootendorst DC, Verduijn M, Elliot EG, Dekker FW, Krediet RT (2010) Performance of the Cockcroft–Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 5:1003–1009
Poli D, Antonucci E, Zanazzi M, Grifoni E, Testa S, Ageno W, Palareti G (2012) Impact of glomerular filtration estimate on bleeding risk in very old patients treated with vitamin K antagonists. Results of EPICA study on the behalf of FCSA Italian Federation of Anticoagulation Clinics. Thromb Haemost 107:1100–1106
Grande D, Gioia MI, Terlizzese P, Iacoviello M (2017) Heart failure and kidney disease. Adv Exp Med Biol. https://doi.org/10.1007/5584_2017_126
Douville P, Martel AR, Talbot J, Desmeules S, Langlois S, Agharazii M (2009) Impact of age on glomerular filtration estimates. Nephrol Dial Transplant 24:97–103
Testa S, Tripodi A, Legnani C, Pengo V, Abbate R, Dellanoce C, Carraro P, Salomone L, Paniccia R, Paoletti O, Poli D, Palareti G, START-Laboratory Register (2016) Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res 137:178–183
Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz MD, Nehmiz G, Wang S, Wallentin L, RE-LY Investigators (2014) The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 63:321–328
Storelli F, Daali Y, Desmeules J, Reny JL, Fontana P (2016) Pharmacogenomics of oral antithrombotic drugs. Curr Pharm Des 22:1933–1949
Tripodi A (2013) The laboratory and the new oral anticoagulants. Clin Chem 59:353–362
Pengo V (2013) Laboratory tests during direct oral anticoagulant treatment? Yes. Intern Emerg Med 8:371–372
Di Minno G, Ricciardi E, Scalera A (2013) Laboratory tests during direct oral anticoagulant treatment? No. Intern Emerg Med 8:367–370
Tripodi A, Chantarangkul V, Legnani C, Testa S, Tosetto A (2018) Inter-laboratory variability for the measurement of direct oral anticoagulants. Results from the external quality assessment scheme. J Thromb Haemost. https://doi.org/10.1111/jth.13949
Marongiu F, Barcellona D (2005) The future of anticoagulation clinics: a journey to thrombosis centers? Haematologica 90:298
Barnes GD, Nallamothu BK, Sales AE, Froehlich JB (2016) Reimagining anticoagulation clinics in the era of direct oral anticoagulants. Circ Cardiovasc Qual Outcomes 9:182–185
Tripodi A, Ageno W, Ciaccio M, Legnani C, Lippi G, Manotti C, Marcucci R, Moia M, Morelli B, Poli D, Steffan A, Testa S (2017) Position paper on laboratory testing for patients on direct oral anticoagulants. Consensus document of SISET, FCSA, SIBioC and SIPMeL. Blood Transf. https://doi.org/10.2450/2017.0124-17
Jaffer IH, Chan N, Roberts R, Fredenburgh JC, Eikelboom JW, Weitz JI (2017) Comparison of the ecarin chromogenic assay and diluted thrombin time for quantification of dabigatran concentrations. J Thromb Haemost 15:2377–2387
Testa S, Legnani C, Tripodi A, Paoletti O, Pengo V, Abbate R, Bassi L, Carraro P, Cini M, Paniccia R, Poli D, Palareti G, For the START-Laboratory Register (2016) Poor comparability of coagulation screening test with specific measurement in patients treated with direct oral anticoagulants: results from a multicenter multiplatform study. J Thromb Haemost 14:2194–2201
Rodgers R, Bagot CN, Lawrence C, Hickman G, McGurk M, Tait RC (2013) Correlating prothrombin time with plasma rivaroxaban level. Br J Haematol 163:685–687
Patel JP, Roberts LN, Chitongo PB, Patel RK, Arya R (2013) More on normal prothrombin times in the presence of therapeutic levels of rivaroxaban-early experience from King’s College Hospital. Br J Haematol 162:717–718
van Veen JJ, Smith J, Kitchen S, Makris M (2013) Normal prothrombin time in the presence of therapeutic levels of rivaroxaban. Br J Haematol 160:859–866
Weitz JI, Eikelboom JW (2016) Urgent need to measure effects of direct oral anticoagulants. Circulation 134:186–188
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Statement on human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
None.
Rights and permissions
About this article
Cite this article
Tripodi, A., Marongiu, F., Moia, M. et al. The vexed question of whether or not to measure levels of direct oral anticoagulants before surgery or invasive procedures. Intern Emerg Med 13, 1029–1036 (2018). https://doi.org/10.1007/s11739-018-1854-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1854-6