Abstract
Purpose of review
Adult survivors of congenital heart disease (CHD) are at increased risk of arrhythmia. The goal of this review is to outline diagnostic and therapeutic approaches to arrhythmia in adult CHD patients.
Recent findings
Macro-reentrant atrial tachyarrhythmia is the most common arrhythmia encountered in adults with CHD. Approximately 25% of hospitalizations associated with arrhythmia. The risk of ventricular arrhythmia is estimated as high as 25–100 times that for the general population and increased after two decades.
Summary
Routine ambulatory monitoring is important for arrhythmia risk assessment in adults with CHD. There are limitations, potential adverse effects, and risk of recurrence with antiarrhythmic drugs, catheter ablation, and surgical approaches. Adults with CHD suffer various forms of arrhythmia, are at increased risk of sudden death, and require special consideration for medical and interventional therapy.
Similar content being viewed by others
Introduction
Adults with congenital heart disease (ACHD) comprise a heterogeneous and burgeoning global population with unique risks for major cardiovascular complications, especially arrhythmia, that confront health care providers and patients with complex and difficult challenges [1, 2]. Advancements in surgery, interventional procedures, and medical care are credited for the increased survival and have resulted in a growing and aging population with adults outnumbering children with congenital heart disease (CHD) in developed nations [1]. Treatment has shifted mortality in CHD towards older ages with a distribution closer to that of the general population [3]. North American epidemiologic data for the last decade report growth in the ACHD population around 60% with substantial increases in the most complex diagnoses [4, 5]. In the USA, there are more than 1.4 million adults with CHD, outnumbering children nearly one- and one-half-fold, with an estimated prevalence up to 6.5 per 1000 [5, 6]. The growth of this population is expected to plateau around the year 2050 and carries implications for the provision of health care given the complex needs and increased risk for complications, including arrhythmia and sudden death [7].
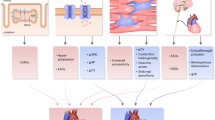
Arrhythmia is increasingly burdensome to patients, providers, and health systems with implications for planning and delivering services to a growing population. Adults with CHD presenting to emergency departments of children’s hospitals have increased, with twice as many having complex diagnoses, and associated with greater risk of admission and death [8]. Figure 1 illustrates factors that contribute to arrhythmic sequelae in CHD including anatomic substrate, malformed or displaced conduction systems, surgical scars, abnormal hemodynamics, fibrosis, and hypoxia [9,10,11, 12•]. ACHD populations suffer various forms and recurrence of arrhythmia, are victim to sudden death, and require special consideration for medical and interventional therapy [13].
Schematic of factors leading to arrhythmias in a pre- and b post-operative congenital heart disease. AV atrioventricular. Reprinted from Canadian Journal of Cardiology, Vol 29 (7), Carolina Escudero MD, FRCPC, Paul Khairy MD, PhD, FRCPC, Shubhayan Sanatani MD, FRCPC, Electrophysiologic Considerations in Congenital Heart Disease and Their Relationship to Heart Failure, Pages 821–829 (2013) with permission from Elsevier.
Bradyarrhythmia
Bradyarrhythmia, whether related to sinus node dysfunction or atrioventricular (AV) block, is common in certain subsets of CHD [9, 12•, 13]. The interaction of congenital anatomic variations, progressive fibrosis, and physical damage during surgery plays a role in the development of bradycardia. Sinus node dysfunction or conduction abnormalities are seen in patients with heterotaxy syndromes, abnormal segmental connections, and post-atrial or ventricular surgery [9, 12•, 13]. Recurrent AV block is recognized as a potential late complication of intervention, particularly following certain ventricular septal defect (VSD) repairs and left ventricular outflow tract operations. Chronotropic incompetence or loss of AV synchrony can cause significant hemodynamic disturbances in patients with atrial baffles or Fontan circulations, leading to symptoms or exercise intolerance, and is poorly tolerated.
Sinus node dysfunction
Congenital heart defects and surgical intervention can contribute to sinus node dysfunction and associated bradycardia [9, 12•]. In heterotaxy syndrome, the sinus node is commonly atypical in morphology, number, or location. Left atrial isomerism with polysplenia heterotaxy is associated with an absent or hypoplastic sinoatrial node that is displaced posterior-inferiorly. In this setting, ectopic atrial bradycardia and junctional rhythms are commonly encountered. Surgical palliation itself can result in direct trauma to the sinoatrial node or artery as in the case of Mustard, Senning, and Fontan procedures [14, 15]. Chronotropic incompetence can result in significant symptoms, negatively impact hemodynamics, and reduce quality of life.
AV block
Displacement of the conduction system is associated with several congenital heart defects such as heterotaxy syndrome, congenitally corrected-transposition of the great arteries (CC-TGA), and endocardial cushion defects [12•]. Absence of a penetrating connection and displacement from the usual course surrounding the triangle of Koch lead to abnormal functional properties of the conduction system. Patients with CC-TGA are at increased risk of congenital and post-surgical heart block with 20% of patients estimated to develop AV block by adulthood [16].
Direct trauma to the conduction system during surgical or catheter-based intervention is a common cause of AV block in CHD patients, occurring in 1–3% of cases [17, 18]. Ventricular septal defect repair, whether isolated or in conjunction with other congenital heart defects, is frequently associated with post-surgical AV block [17]. Post-surgical complete heart block is reported to occur in 4–8% of CHD repairs involving VSDs [19]. The subsequent need for chronic right ventricular pacing can lead to inter-ventricular dyssynchrony, remodeling, and systolic dysfunction [20].
Tachyarrhythmia
Macro-reentrant atrial tachyarrhythmia is the most common arrhythmia encountered in adults with CHD [21, 22]. Atrial arrhythmia in CHD patients is three times more prevalent than in the general population, with a cumulative lifetime risk near 50%, higher with greater age and CHD complexity, and associated with adverse outcomes including heart failure, death, and need for intervention [23]. Intra-atrial reentrant tachycardia (IART) is the most common atrial arrhythmia, with the prevalence of atrial fibrillation (AF) surpassing IART at age 50, and increasingly permanent at older ages [24]. The risk of developing AF in setting of CHD is 22 times higher than the general population, greatest with the most complex diagnoses, and increased 3.6-fold with history of surgery [25]. Cavotricuspid isthmus (CTI)-related IART is most frequently encountered, although non-CTI IART is often seen concomitantly or alone and related to CHD complexity [26]. Adult CHD patients with atrial arrhythmia have a 2-fold increased risk of death and a 4-fold increased risk of heart failure [27]. Atrial arrhythmia with 1:1 AV conduction may contribute to and is poorly tolerated in setting of ventricular dysfunction.
The incidence of ventricular arrhythmia in ACHD patients is estimated at 0.1–0.2% per year [13]. The primary mechanism is reentry involving ventriculotomy incision or scar tissue [28]. Ventricular arrhythmias are thought to be responsible for most sudden cardiac deaths (SCD) in ACHD, for which the risk is estimated as high as 25–100 times that for the general population and increased after two decades [29]. SCD is cited as the most frequent cause of mortality in ACHD [30,31,32]. Recent studies estimate that 23% of deaths in ACHD patients are due to sudden cardiac death [30, 31]. Guideline recommendations exist for management of ventricular arrhythmia [13, 33, 34, 35•]. It is recognized that the clinical prognostic utility of ventricular ectopy and non-sustained or sustained ventricular tachycardia (VT) varies depending on the underlying CHD, with increased risk in patients with Eisenmenger syndrome, CC-TGA, D-TGA post-atrial switch, and Fontan circulation [36].
Several studies suggest an association of atrial arrhythmias and QRS prolongation with increased risk of SCD [36,37,38]. Additional predictive factors include ventricular dysfunction [37], AV valve replacement, and post-bypass Fontan pressure > 20 mmHg [38]. In patients with pulmonary arterial hypertension in the setting of CHD, a prior history of arrhythmia, predominately supraventricular arrhythmia, was associated with death [39].
Diagnostic evaluation
Non-invasive tests remain critical to the evaluation of arrhythmia in ACHD patients. The 2018 ACHD guidelines provide direction in the frequency recommended for such evaluation [35•]. The 12-lead ECG remains an important diagnostic tool that can also direct subsequent therapy. Routine ambulatory monitoring is recommended in asymptomatic patients that are increased risk of conduction disease and arrhythmia. Disease-specific recommendations exist for routine surveillance in patients with Tetralogy of Fallot (ToF), CC-TGA, D-TGA, and Fontan physiology [35•]. Event monitors may provide additional information in symptomatic patients.
Continued monitoring with implantable loop recorders (ILR) are particularly useful in patients with syncope, recurrent palpitations, or episodes that have been unable to be captured on traditional monitors. Recent data demonstrate that ILRs provide useful clinical information in over half of the patients evaluated for syncope with a median time to diagnosis of 4.5 months [40].
Diagnostic electrophysiology (EP) study is useful in risk assessment in CHD such as repaired ToF thought to be at risk for VT and SCD and should be considered in several scenarios in the ACHD population including unexplained syncope and palpitations suggestive of tachyarrhythmia [13]. In CHD patients with ventricular pre-excitation, studies are recommended for risk stratification even if asymptomatic. Patients who may be undergoing cardiac surgery, especially if catheter access will be limited post-operatively, should be considered for EP study pre-operatively.
Therapeutic options
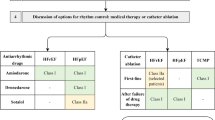
A series of guidelines for the evaluation and treatment of ventricular arrhythmias and prevention of sudden cardiac death have been published, most recently by a collaborative effort of the American Heart Association, American College of Cardiology, and Heart Rhythm Society [33] and by the European Society of Cardiology [34]. Similar guidelines have been published for the management of supraventricular tachycardia [41]. While comprehensive in terms of the broad spectrum of arrhythmias and available treatments, these guidelines include little information specific to CHD in adult patients. A collaborative statement by Pediatric and Congenital Electrophysiology Society and HRS in 2014 directly addressed arrhythmia management in ACHD patients [13].
Medical therapy
Choice of antiarrhythmic therapy in adults with CHD should be made in consideration of co-existing conduction disease, ventricular dysfunction, and non-cardiac comorbidities [13]. Class IC antiarrhythmic drugs (flecainide and propafenone) may facilitate VT by decreasing conduction and increasing spatially heterogeneous action potential duration in setting of incisional scarring and fibrosis. Due to their proarrhythmic effects, class 1C antiarrhythmics are contraindicated in patients with conduction abnormalities, structural heart disease, and ventricular dysfunction [42, 43]. In a recent retrospective study, flecainide was found to be equally effective in pediatric patients with and without CHD with no difference in the rate of adverse events [44]. There is sparse data regarding the safety and efficacy of flecainide in ACHD patients.
QT prolongation and ventricular arrhythmias including torsades de pointes are important side effects of class III antiarrhythmic drugs. Amiodarone has been associated with other significant adverse effects, including thyroid dysfunction that can in turn exacerbate arrhythmia [45]. While amiodarone is highly effective, toxicity and intolerance lead to a high rate of discontinuation. Low-dose amiodarone (≤ 200 mg/day) was found to be effective for control of tachyarrhythmia with a low incidence of adverse effects in a select group of CHD patients [46]. Similar to amiodarone, sotalol has been shown to be effective in controlling arrhythmia in adults with CHD, but nearly 20% of patients discontinue sotalol secondary to adverse effects. Patients with Fontan physiology are particularly affected by significant bradycardia, similar to that seen with amiodarone [45, 47]. For acute pharmacologic conversion of IART, ibutilide has been shown to successfully convert > 70% of cases [48]. Dofetilide also demonstrates adequate rhythm control, but is associated with significant adverse events involving ventricular arrhythmia [49].
With consideration of potential adverse effects, overall data would suggest use of amiodarone or dofetilide in setting of complex CHD or systemic ventricular dysfunction [13, 50]. Dofetilide requires close monitoring of concomitant medications, QT interval, and renal function as it is contraindicated in patients with renal failure. Amiodarone requires close monitoring of thyroid, hepatic, and pulmonary function and should be used with caution in patients with cyanotic heart disease, low body mass index, or QT prolongation [13]. The addition of AV nodal blocking medications such as beta blockers or non-dihydropyridine calcium channel blockers (verapamil, diltiazem) may help to prevent rapid ventricular rate, associated symptoms, and cardiac impairment.
Catheter ablation
Adverse effects of antiarrhythmic medications, their limitations, and patient choice for a drug-free lifestyle contribute to increased utility of catheter ablation. Catheter ablation is a common treatment for arrhythmias with an average acute success rate of 75% [51, 52]. While acutely effective, several studies demonstrate a higher recurrence rate in adults with CHD [53, 54] ranging from 12 h to 4–5 year [55]. Body mass index ≥ 30 and Fontan circulation have been associated with arrhythmia recurrence post-ablation [55].
While IART is the most common arrhythmia encountered overall in adults with CHD, AF has emerged as the most common arrhythmia in those aged greater than 50 years [24] with an incidence of 4–15%, which is considerably higher than in the general population. Incisional scars that may predispose to re-entrant circuits, atrial dilation and subsequent fibrosis, and activation of different atrial sites in the setting of sinus node dysfunction are proposed mechanisms that may facilitate initiation and perpetuation of AF. Catheter ablation of AF is acutely successful in 72% of cases [56] with at least partial long-term freedom from AF in follow-up regardless of CHD complexity [57]. Ablation of IART demonstrates similar acute success rate of 75–78% [58, 59]. A recent study suggests that acute success is a predictor of freedom from recurrence [60], which has been associated with previous AF, non-CTI IART, and complex CHD [58, 59].
Congenital displacement and anomalous course of the conduction system in certain CHD lesions can disrupt the traditional location of the AV node relative to the triangle of Koch, which is important in the catheter ablation of AV nodal reentry tachycardia (AVNRT). Recent studies report a success rate of 86–100% of AVNRT cases [61, 62]. Complex CHD was associated with longer procedure time and increased risk of AV block [61]. It is reasonable to defer if AV nodal location is uncertain.
Ventricular arrhythmia is not uncommon in adults with CHD and the prevalence is higher in patients with VSD patch closure or ventricular incision [63]. The primary mechanism is reentry involving a critical isthmus [64, 65]. In ventricular arrhythmia, catheter ablation is successful in greater than 80% of cases, with a recurrence rate of 14% [66]. Confirmation of conduction block is predictive of preventing recurrence [66]. High-density mapping, contact force, irrigated tip catheters, and intracardiac echocardiography are additional strategies that may help to improve ablation outcomes.
Device therapy
There is an increasing need for implantable cardiac devices in ACHD patients due to the high prevalence of congenital and post-operative sinus node dysfunction and AV block. Chronic isolated sinus node dysfunction or loss of AV synchrony contributes to impaired hemodynamics. Symptomatic sinus node dysfunction and symptomatic bradycardia with concomitant AV block are class I indications for pacing in ACHD patients [13]. As sinus bradycardia increases the interval during which a premature atrial complex may initiate IART, consideration can be made for pacemaker implantation to prevent recurrent tachyarrhythmia [67]. Devices with antitachycardia pacing have been found to be effective in terminating IART in ACHD patients [68, 69].
Lead position is an important aspect of pre-procedural planning. Traditional atrial appendage placement may not be feasible in certain post-repair cases. In addition, compared to appendage pacing, atrial septal position may decrease the frequency of unnecessary ventricular pacing [70]. While there is no evident single optimal endocardial ventricular pacing site, several studies have demonstrated that the right ventricular free wall should be avoided [71, 72].
Sudden cardiac death accounts for 20–25% of deaths in CHD patients post-surgery [30, 73], with the majority related to arrhythmia [73]. Patients at higher risk include those with CC-TGA, D-TGA post-Mustard or Senning, and ToF [29, 73]. There are additional elements in ToF that have been associated with increased risk including increased QRS duration, non-sustained ventricular tachycardia, and inducible sustained ventricular tachycardia [21, 74], particularly in those with history of ventriculotomy. In this population, there is an 8–10% annual rate of appropriate ICD shocks [74].
Whether in pacing or ICD systems, transvenous leads are to be avoided in the setting of intracardiac shunts [75]. The subcutaneous ICD (S-ICD) has emerged as an alternative to the transvenous ICD system. The electrode is placed in the left parasternal position and the pulse generator in the left mid-axillary line. The S-ICD implantation method offers the advantage of minimizing procedure-related complications, preserving venous structures, and providing an alternative route when transvenous access to the ventricular is precluded by abnormal systemic venous pathways as in the setting of complex CHD. Eligibility ECG screening is performed to avoid oversensing of the T-wave. Abnormal ECG findings associated with ineligibility include T-wave inversion, prolonged QRS duration, and insufficient R:T wave ratio [76, 77]. Approximately 75% of ACHD patients meet eligibility criteria with at least one suitable vector [78, 79], with potential increase in eligibility rate with right parasternal lead position [80]. As the S-ICD system does not allow for conventional pacing or ATP, it can be considered reasonable in ACHD patients that do not have bradycardia or require ATP functionality.
Adults with CHD are at increased risk for complications related to implantable cardiac devices including infection, device malfunction, lead malfunction, and venous occlusion [81,82,83] that may lead to a need for lead abandonment or extraction. Up to 30% of ACHD patients will have at least one or more abandoned transvenous lead [84]. Transvenous lead extraction has inherent risks and complications in patients with structurally normal hearts [85]. In the ACHD population, 40% of lead extractions are successfully performed with simple extraction [84] and greater than 90% are completely extracted with advanced extraction techniques including those with lumenless pacing leads [86, 87]. While transvenous lead extraction has been performed with similar complication rates as that in adult patients without CHD [84, 88], careful consideration of the risks and benefits must be weighed regarding whether to abandon or remove the lead. While intended to be life-saving, implanted cardiac rhythm devices can be associated with depression, increased anxiety, and decreased quality of life [89, 90], highlighting the potential utility of appropriate screening prior to implantation.
Surgical therapy
Many ACHD patients require reoperation for deterioration of implanted prostheses or residual hemodynamic sequelae, creating an opportunity to surgically address substrates for arrhythmia [13, 91,92,93]. Surgical techniques for arrhythmia emerged prior to the development of transcatheter options, were pioneered for supraventricular arrhythmias, and were initially utilized in patients without CHD [94, 95]. Early approaches were developed for treatment of accessory pathways, atrial flutter, and then atrial fibrillation using cut-and-sew, energy ablation, or combined techniques (e.g., Cox-Maze procedure). Operative antiarrhythmic strategies have been adapted for CHD and refined for use across the spectrum of anatomic and electrical heterogeneity [91, 92]. Recommendations have been developed for operative management of arrhythmia in ACHD, yet the scope is not comprehensive and approaches to treatment and prophylaxis continue to evolve [13, 35•, 96].
Atrial arrhythmia is most prevalent in certain right heart anomalies, univentricular hearts, and following atrial surgery [13, 96]. Substrates for AF frequently reside in the left atrium, and the aim of left atrial surgery is isolation of the pulmonary veins and disruption of critical isthmuses for reentry (left AV valve, coronary sinus, and Bachmann’s bundle), often including left atrial appendage ligation [97]. Right atrial macro-reentrant tachycardias (IART, AF) can occur around conduction barriers that are common from prior operation (e.g., atrial incisions, patches, baffles) and often involve the CTI. Successful operative approaches must create transmural lines of conduction block that completely isolate arrhythmic foci or areas of slow conduction; otherwise, any gap may serve as a nidus for recurrent arrhythmia [91, 93, 96].
Arrhythmia surgery in ACHD is safe and effective with favorable results [98,99,100,101,102]. Cox-Maze type procedures (right, left, and biatrial) were predominantly utilized with modifications to account for specific anatomic or prior surgical factors, and complications or mortality related to the arrhythmia surgery were very low or absent. High success rates and freedom from recurrence of up to 75% at 6 years are reported, although age, arrhythmia type, and duration are related to recurrence (e.g., persistent AF) and may represent targets for earlier or modified intervention [98,99,100,101,102]. Specifically considering Fontan conversion surgery, durable long-term freedom from late recurrence of atrial tachycardia is 77% at 10 years with no recurrence of atrial fibrillation after biatrial arrhythmia surgery [103].
There is considerably higher risk of ventricular arrhythmia and SCD in patients with ToF, systemic right ventricle, and left heart obstruction [13]. Intraoperative treatment of VT most often accompanies surgery for hemodynamic lesions, with best practices still evolving. Operative techniques range from cryoablation to endocardial/epicardial resection, with success rates from 50 to 85% and frequently combined with defibrillator implantation given the risks and consequences of treatment failure [13]. Although risk scoring has not been prospectively validated, recent data has emerged regarding the use of perioperative EP studies in patients with ToF at risk for VT after pulmonary valve replacement (PVR) [104, 105]. In an unselected adult population undergoing PVR, 49% had inducibility before surgery, 47% remained inducible afterward and underwent defibrillator implantation, and 21% of those received appropriate ICD shocks for symptomatic VT [104]. These results highlight the high rate of inducibility in patients with ToF, how surgical ablative therapy may reduce the rate of potentially malignant arrhythmia, and underscore the importance of patient selection in surgical ablation.
Conclusion
Adult survivors of CHD are vulnerable to hemodynamic compromise and decreased quality of life secondary to paroxysmal and at times life-threatening arrhythmia. Routine monitoring and advanced therapeutic options may serve to decrease morbidity and mortality in these patients.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Webb G, Mulder BJ, Aboulhosn J, Daniels CJ, Elizari MA, Hong G, et al. The care of adults with congenital heart disease across the globe: current assessment and future perspective: a position statement from the International Society for Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2015;195:326–33.
Ntiloudi D, Giannakoulas G, Parcharidou D, Panagiotidis T, Gatzoulis MA, Karvounis H. Adult congenital heart disease: a paradigm of epidemiological change. Int J Cardiol. 2016;218:269–74.
Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56(14):1149–57.
Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–56.
Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134(2):101–9.
Raskind-Hood C, Hogue C, Overwyk KJ, Book W. Estimates of adolescent and adult congenital heart defect prevalence in metropolitan Atlanta, 2010, using capture-recapture applied to administrative records. Ann Epidemiol. 2019;32:72–7 e72.
Benziger CP, Stout K, Zaragoza-Macias E, Bertozzi-Villa A, Flaxman AD. Projected growth of the adult congenital heart disease population in the United States to 2050: an integrative systems modeling approach. Popul Health Metrics. 2015;13:29.
Mohan S, Moffett BS, Lam W, de la Uz C, Miyake C, Valdes SO, et al. Analysis of adults with congenital heart disease presenting to pediatric emergency departments with arrhythmias. Congenit Heart Dis. 2017;12(4):507–11.
Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115(4):534–45.
Janson CM, Shah MJ. Supraventricular tachycardia in adult congenital heart disease: mechanisms, diagnosis, and clinical aspects. Card Electrophysiol Clin. 2017;9(2):189–211.
Sathananthan G, Harris L, Nair K. Ventricular arrhythmias in adult congenital heart disease: mechanisms, diagnosis, and clinical aspects. Card Electrophysiol Clin. 2017;9(2):213–23.
• Moore JP, Aboulhosn JA. Introduction to the congenital heart defects: anatomy of the conduction system. Card Electrophysiol Clin. 2017;9(2):167–75 This article summarizes important variation in conduction system anatomy across the spectrum of congenital heart disease lesions.
Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11(10):e102–65.
Bossers SS, Duppen N, Kapusta L, et al. Comprehensive rhythm evaluation in a large contemporary Fontan population. Eur J Cardiothorac Surg. 2015;48(6):833–40 discussion 840-831.
Lasa JJ, Glatz AC, Daga A, Shah M. Prevalence of arrhythmias late after the Fontan operation. Am J Cardiol. 2014;113(7):1184–8.
Beauchesne LM, Warnes CA, Connolly HM, Ammash NM, Tajik AJ, Danielson GK. Outcome of the unoperated adult who presents with congenitally corrected transposition of the great arteries. J Am Coll Cardiol. 2002;40(2):285–90.
Siehr SL, Hanley FL, Reddy VM, Miyake CY, Dubin AM. Incidence and risk factors of complete atrioventricular block after operative ventricular septal defect repair. Congenit Heart Dis. 2014;9(3):211–5.
Lin A, Mahle WT, Frias PA, Fischbach PS, Kogon BE, Kanter KR, et al. Early and delayed atrioventricular conduction block after routine surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2010;140(1):158–60.
Anderson JB, Czosek RJ, Knilans TK, Meganathan K, Heaton P. Postoperative heart block in children with common forms of congenital heart disease: results from the KID Database. J Cardiovasc Electrophysiol. 2012;23(12):1349–54.
Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13(12):2272–8.
Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon F̧P, Kay J, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122(9):868–75.
Wasmer K, Kobe J, Dechering DG, et al. Isthmus-dependent right atrial flutter as the leading cause of atrial tachycardias after surgical atrial septal defect repair. Int J Cardiol. 2013;168(3):2447–52.
Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120(17):1679–86.
Labombarda F, Hamilton R, Shohoudi A, Aboulhosn J, Broberg CS, Chaix MA, et al. Increasing prevalence of atrial fibrillation and permanent atrial arrhythmias in congenital heart disease. J Am Coll Cardiol. 2017;70(7):857–65.
Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Gilljam T, Hansson PO, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137(9):928–37.
Roca-Luque I, Rivas-Gandara N, Subira LD, et al. Mechanisms of intra-atrial re-entrant tachycardias in congenital heart disease: types and predictors. Am J Cardiol. 2018;122(4):672–82.
Yang H, Kuijpers JM, de Groot JR, Konings TC, van Dijk A, Sieswerda GT, et al. Impact of atrial arrhythmias on outcome in adults with congenital heart disease. Int J Cardiol. 2017;248:152–4.
Zeppenfeld K, Schalij MJ, Bartelings MM, Tedrow UB, Koplan BA, Soejima K, et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116(20):2241–52.
Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32(1):245–51.
Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86(10):1111–6.
Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31(10):1220–9.
Naidu P, Grigg L, Zentner D. Mortality in adults with congenital heart disease. Int J Cardiol. 2017;245:125–30.
Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91–e220.
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015;17(11):1601–87.
Stout KK, Daniels CJ, Aboulhosn JA, et al. AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):1494–1563. This article summarizes disease-specific recommendations regarding surveillance and management of arrhythmia in adults with congenital heart disease.
Moore B, Yu C, Kotchetkova I, Cordina R, Celermajer DS. Incidence and clinical characteristics of sudden cardiac death in adult congenital heart disease. Int J Cardiol. 2018;254:101–6.
Koyak Z, de Groot JR, Bouma BJ, Zwinderman AH, Silversides CK, Oechslin EN, et al. Sudden cardiac death in adult congenital heart disease: can the unpredictable be foreseen? Europace. 2017;19(3):401–6.
Pundi KN, Pundi KN, Johnson JN, Dearani JA, Li Z, Driscoll DJ, et al. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis. 2017;12(1):17–23.
Drakopoulou M, Nashat H, Kempny A, Alonso-Gonzalez R, Swan L, Wort SJ, et al. Arrhythmias in adult patients with congenital heart disease and pulmonary arterial hypertension. Heart. 2018;104(23):1963–9.
Bezzerides VJ, Walsh A, Martuscello M, Escudero CA, Gauvreau K, Lam G, et al. The real-world utility of the LINQ implantable loop recorder in pediatric and adult congenital heart patients. JACC Clin Electrophysiol. 2019;5(2):245–51.
Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):e27–e115.
Epstein AE, Hallstrom AP, Rogers WJ, Liebson PR, Seals AA, Anderson JL, et al. Mortality following ventricular arrhythmia suppression by encainide, flecainide, and moricizine after myocardial infarction. The original design concept of the Cardiac Arrhythmia Suppression Trial (CAST). JAMA. 1993;270(20):2451–5.
Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102(7):748–54.
Cunningham T, Uzun O, Morris R, Franciosi S, Wong A, Jeremiasen I, et al. The safety and effectiveness of flecainide in children in the current era. Pediatr Cardiol. 2017;38(8):1633–8.
Moore BM, Cordina RL, McGuire MA, Celermajer DS. Adverse effects of amiodarone therapy in adults with congenital heart disease. Congenit Heart Dis. 2018;13(6):944–51.
Iwasawa S, Uyeda T, Saito M, Ishii T, Inage A, Hamamichi Y, et al. Efficacy and safety of low-dose amiodarone therapy for tachyarrhythmia in congenital heart disease. Pediatr Cardiol. 2018;39(5):1016–22.
Moore BM, Cordina RL, McGuire MA, Celermajer DS. Efficacy and adverse effects of sotalol in adults with congenital heart disease. Int J Cardiol. 2019;274:74–9.
Hoyer AW, Balaji S. The safety and efficacy of ibutilide in children and in patients with congenital heart disease. Pacing Clin Electrophysiol. 2007;30(8):1003–8.
El-Assaad I, Al-Kindi SG, Abraham J, et al. Use of dofetilide in adult patients with atrial arrhythmias and congenital heart disease: a PACES collaborative study. Heart Rhythm. 2016;13(10):2034–9.
Kumar S, Tedrow UB, Triedman JK. Arrhythmias in adult congenital heart disease: diagnosis and management. Cardiol Clin. 2015;33(4):571–88 viii.
Collins KK, Love BA, Walsh EP, Saul JP, Epstein MR, Triedman JK. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000;86(9):969–74.
Kanter RJ, Papagiannis J, Carboni MP, Ungerleider RM, Sanders WE, Wharton JM. Radiofrequency catheter ablation of supraventricular tachycardia substrates after mustard and senning operations for d-transposition of the great arteries. J Am Coll Cardiol. 2000;35(2):428–41.
Triedman JK, Alexander ME, Love BA, Collins KK, Berul CI, Bevilacqua LM, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39(11):1827–35.
Triedman JK, Bergau DM, Saul JP, Epstein MR, Walsh EP. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30(4):1032–8.
Lewis M, Whang W, Biviano A, Hickey K, Garan H. Rosenbaum M. Congenit Heart Dis: Predictors and rates of recurrence of atrial arrhythmias following catheter ablation in adults with congenital heart disease; 2018.
Yap SC, Harris L, Downar E, Nanthakumar K, Silversides CK, Chauhan VS. Evolving electroanatomic substrate and intra-atrial reentrant tachycardia late after Fontan surgery. J Cardiovasc Electrophysiol. 2012;23(4):339–45.
Liang JJ, Frankel DS, Parikh V, et al. Safety and outcomes of catheter ablation for atrial fibrillation in adults with congenital heart disease: a multicenter registry study. Heart Rhythm. 2019;16(6):846–852.
Roca-Luque I, Rivas-Gandara N, Dos Subira L, et al. Long-term follow-up after ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease: types and predictors of recurrence. JACC Clin Electrophysiol. 2018;4(6):771–80.
Roca-Luque I, Rivas-Gandara N, Dos-Subira L, et al. Predictors of acute failure ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease: cardiac disease, atypical flutter, and previous atrial fibrillation. J Am Heart Assoc. 2018;7(7).
Grubb CS, Lewis M, Whang W, Biviano A, Hickey K, Rosenbaum M, et al. Catheter ablation for atrial tachycardia in adults with congenital heart disease: electrophysiological predictors of acute procedural success and post-procedure atrial tachycardia recurrence. JACC Clin Electrophysiol. 2019;5(4):438–47.
Papagiannis J, Beissel DJ, Krause U, et al. Atrioventricular nodal reentrant tachycardia in patients with congenital heart disease: outcome after catheter ablation. Circ Arrhythm Electrophysiol. 2017;10(7).
Upadhyay S, Marie Valente A, Triedman JK, Walsh EP. Catheter ablation for atrioventricular nodal reentrant tachycardia in patients with congenital heart disease. Heart Rhythm. 2016;13(6):1228–37.
Brouwer C, Hazekamp MG, Zeppenfeld K. Anatomical substrates and ablation of reentrant atrial and ventricular tachycardias in repaired congenital heart disease. Arrhythmia Electrophysiol Rev. 2016;5(2):150–60.
Yang J, Brunnquell M, Liang JJ, Callans DJ, Garcia FC, Lin D, et al. Long term follow up after ventricular tachycardia ablation in patients with congenital heart disease. J Cardiovasc Electrophysiol. 2019;1–9.
Kapel GF, Reichlin T, Wijnmaalen AP, et al. Re-entry using anatomically determined isthmuses: a curable ventricular tachycardia in repaired congenital heart disease. Circ Arrhythm Electrophysiol. 2015;8(1):102–9.
van Zyl M, Kapa S, Padmanabhan D, et al. Mechanism and outcomes of catheter ablation for ventricular tachycardia in adults with repaired congenital heart disease. Heart Rhythm. 2016;13(7):1449–54.
Silka MJ, Manwill JR, Kron J, McAnulty JH. Bradycardia-mediated tachyarrhythmias in congenital heart disease and responses to chronic pacing at physiologic rates. Am J Cardiol. 1990;65(7):488–93.
Kamp AN, LaPage MJ, Serwer GA, Dick M 2nd, Bradley DJ. Antitachycardia pacemakers in congenital heart disease. Congenit Heart Dis. 2015;10(2):180–4.
Kramer CC, Maldonado JR, Olson MD, Gingerich JC, Ochoa LA, Law IH. Safety and efficacy of atrial antitachycardia pacing in congenital heart disease. Heart Rhythm. 2018;15(4):543–7.
Acosta H, Viafara LM, Izquierdo D, Pothula VR, Bear J, Pothula S, et al. Atrial lead placement at the lower atrial septum: a potential strategy to reduce unnecessary right ventricular pacing. Europace. 2012;14(9):1311–6.
Janousek J. Device therapy in children with and without congenital heart disease. Herzschrittmacherther Elektrophysiol. 2014;25(3):183–7.
Janousek J, van Geldorp IE, Krupickova S, et al. Permanent cardiac pacing in children: choosing the optimal pacing site: a multicenter study. Circulation. 2013;127(5):613–23.
Koyak Z, Harris L, de Groot JR, Silversides CK, Oechslin EN, Bouma BJ, et al. Sudden cardiac death in adult congenital heart disease. Circulation. 2012;126(16):1944–54.
Khairy P, Harris L, Landzberg MJ, Viswanathan S, Barlow A, Gatzoulis MA, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117(3):363–70.
Khairy P, Landzberg MJ, Gatzoulis MA, Mercier LA, Fernandes SM, Côté JM, et al. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation. 2006;113(20):2391–7.
Groh CA, Sharma S, Pelchovitz DJ, Bhave PD, Rhyner J, Verma N, et al. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2014;11(8):1361–6.
Olde Nordkamp LRA, Warnaars JLF, Kooiman KM, et al. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS-T-wave morphology screening. J Cardiovasc Electrophysiol. 2014;25(5):494–9.
Garside H, Leyva F, Hudsmith L, Marshall H, de Bono J. Eligibility for subcutaneous implantable cardioverter defibrillators in the adult congenital heart disease population. Pacing Clin Electrophysiol. 2019;42(1):65–70.
Zeb M, Curzen N, Veldtman G, Yue A, Roberts P, Wilson D, et al. Potential eligibility of congenital heart disease patients for subcutaneous implantable cardioverter-defibrillator based on surface electrocardiogram mapping. Europace. 2015;17(7):1059–67.
Okamura H, McLeod CJ, DeSimone CV, et al. Right parasternal lead placement increases eligibility for subcutaneous implantable cardioverter defibrillator therapy in adults with congenital heart disease. Circ J. 2016;80(6):1328–35.
Santharam S, Hudsmith L, Thorne S, Clift P, Marshall H, De Bono J. Long-term follow-up of implantable cardioverter-defibrillators in adult congenital heart disease patients: indications and outcomes. Europace. 2017;19(3):407–13.
Hayward RM, Dewland TA, Moyers B, Vittinghoff E, Tanel RE, Marcus GM, et al. Device complications in adult congenital heart disease. Heart Rhythm. 2015;12(2):338–44.
Midha D, Chen Z, Jones DG, Williams HJ, Lascelles K, Jarman J, et al. Pacing in congenital heart disease - a four-decade experience in a single tertiary centre. Int J Cardiol. 2017;241:177–81.
Fender EA, Killu AM, Cannon BC, Friedman PA, Mcleod CJ, Hodge DO, et al. Lead extraction outcomes in patients with congenital heart disease. Europace. 2017;19(3):441–6.
Wazni O, Epstein LM, Carrillo RG, Love C, Adler SW, Riggio DW, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55(6):579–86.
Shepherd E, Stuart G, Martin R, Walsh MA. Extraction of SelectSecure leads compared to conventional pacing leads in patients with congenital heart disease and congenital atrioventricular block. Heart Rhythm. 2015;12(6):1227–32.
Garnreiter J, Whitaker P, Pilcher T, Etheridge S, Saarel E. Lumenless pacing leads: performance and extraction in pediatrics and congenital heart disease. Pacing Clin Electrophysiol. 2015;38(1):42–7.
Gourraud JB, Chaix MA, Shohoudi A, et al. Transvenous lead extraction in adults with congenital heart disease: insights from a 20-year single-center experience. Circ Arrhythm Electrophysiol. 2018;11(2):e005409.
Bedair R, Babu-Narayan SV, Dimopoulos K, Quyam S, Doyle AM, Swan L, et al. Acceptance and psychological impact of implantable defibrillators amongst adults with congenital heart disease. Int J Cardiol. 2015;181:218–24.
Cook SC, Valente AM, Maul TM, et al. Shock-related anxiety and sexual function in adults with congenital heart disease and implantable cardioverter-defibrillators. Heart Rhythm. 2013;10(6):805–10.
Mavroudis C, Deal BJ. Prophylactic arrhythmia surgery in association with congenital heart disease. Transl Pediatr. 2016;5(3):148–59.
Mavroudis C, Deal B, Backer CL, Stewart RD. Operative techniques in association with arrhythmia surgery in patients with congenital heart disease. World J Pediatr Congenit Heart Surg. 2013;4(1):85–97.
Uemura H. Surgical aspects of atrial arrhythmia : right atrial ablation and anti-arrhythmic surgery in congenital heart disease. Herzschrittmacherther Elektrophysiol. 2016;27(2):137–42.
Cox JL. Cardiac surgery for arrhythmias. Heart Rhythm. 2004;1(5 Suppl):85C–101C.
Lawrance CP, Henn MC, Damiano RJ Jr. Surgery for atrial fibrillation. Heart Fail Clin. 2016;12(2):235–43.
Deal BJ, Mavroudis C. Arrhythmia surgery for adults with congenital heart disease. Card Electrophysiol Clin. 2017;9(2):329–40.
Mavroudis C, Stulak JM, Ad N, Siegel A, Giamberti A, Harris L, et al. Prophylactic atrial arrhythmia surgical procedures with congenital heart operations: review and recommendations. Ann Thorac Surg. 2015;99(1):352–9.
Gutierrez SD, Earing MG, Singh AK, Tweddell JS, Bartz PJ. Atrial tachyarrhythmias and the Cox-maze procedure in congenital heart disease. Congenit Heart Dis. 2013;8(5):434–9.
Gonzalez Corcia MC, Walsh EP. Emani S. Europace: Long-term results of atrial maze surgery in patients with congenital heart disease; 2019.
Ramdjan T, Mouws E, Kik C, Roos-Hesselink JW, Bogers A, de Groot NMS. Concomitant arrhythmia surgery in patients with congenital heart disease. Interact Cardiovasc Thorac Surg. 2018;27(6):902–9.
Sakamoto SI, Hiromoto A, Ishii Y, Sasaki T, Miyagi Y, Nitta T. Surgical outcomes of modified-maze procedures in adults with atrial septal defect. Surg Today. 2019;49(2):124–9.
Giamberti A, Pluchinotta FR, Chessa M, et al. Surgery for supraventricular tachycardia and congenital heart defects: long-term efficacy of the combined approach in adult patients. Europace. 2017;19(9):1542–8.
Deal BJ, Costello JM, Webster G, Tsao S, Backer CL, Mavroudis C. Intermediate-term outcome of 140 consecutive Fontan conversions with arrhythmia operations. Ann Thorac Surg. 2016;101(2):717–24.
Sandhu A, Ruckdeschel E, Sauer WH, Collins KK, Kay JD, Khanna A, et al. Perioperative electrophysiology study in patients with tetralogy of Fallot undergoing pulmonary valve replacement will identify those at high risk of subsequent ventricular tachycardia. Heart Rhythm. 2018;15(5):679–85.
Caldaroni F, Lo Rito M, Chessa M, et al. Surgical ablation of ventricular tachycardia in patients with repaired tetralogy of Fallot. Eur J Cardiothorac Surg. 2019;55(5):845–850.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Arrhythmia
Rights and permissions
About this article
Cite this article
Guerrier, K., Hendrickson, B., Waller, B.R. et al. Diagnostic and Therapeutic Approach to Arrhythmias in Adult Congenital Heart Disease. Curr Treat Options Cardio Med 21, 44 (2019). https://doi.org/10.1007/s11936-019-0749-9
Published:
DOI: https://doi.org/10.1007/s11936-019-0749-9