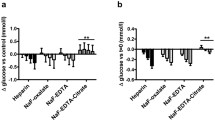
Abstract
Status of diabetes of an individual is majorly evaluated by the frequent monitoring of glucose estimation. Use of serum samples and inappropriate plasma for estimating glucose is an existing practise in Indian standard of laboratories. There is a strong evidence for occurrence of in vitro glycolysis on the above mentioned specimens. The aim is to study the pre-analytical variations on the glucose estimation of using sodium fluoride–disodium EDTA (NaF–Na2EDTA) plasma (glycolysis inhibiting anticoagulant) and determine the fact behind the activity of glycolysis inhibition on the same. Healthy volunteers 20–35 years of both genders consisting of 40 members were selected for the study, and after getting the informed consent form, random blood samples were collected to study the errors of pre-analytical i.e., mixing of NaF–Na2EDTA tube by phlebotomist (no of inversion). Difference in duration from blood collection to centrifugation and a variable in time were taken from centrifugation to analyzing the plasma sample. Comparative studies on EDTA plasma and serum sample were also carried out. The usage of the evacuated blood collection system on NaF–Na2EDTA was shown to have the complete glycolysis inhibitor among all pre-analytical errors, whereas other tubes shown considerable increase in glycolysis. Recently the use of glycolysis inhibitor tubes are come into practise only in accredited or certified laboratories. Hence the authentication of glycolysis inhibition study is mandatory for the pre-analytical variations on the same.






Similar content being viewed by others
References
National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–57.
Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19(9):708–23.
Bergman RN, Finegood DT, Kahn SE. The evolution of β-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Investig. 2002;32(s3):35–45.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62.
American Diabetes Association. 2 Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–16.
World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy. Geneva: World Health Organization; 2010.
Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics-e-book. Amsterdam: Elsevier Health Sciences; 2012.
De Mello VD, Lindström J, Eriksson J, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Sundvall J, et al. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: the Finnish Diabetes Prevention Study. Diabetes Care. 2012;35(2):211–7.
American Diabetes Association. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–14.
Chan AY, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem. 1989;35(2):315–7.
Bruns DE, Knowler WC. Stabilization of glucose in blood samples: why it matters. Clin Chem. 2009;55:850–2.
Meites S, Saniel-Banrey K. Preservation, distribution, and assay of glucose in blood, with special reference to the newborn. Clin Chem. 1979;25(4):531–4.
Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem. 2007;53(12):2040–1.
Uchida K, Matuse R, Toyoda E, Okuda S, Tomita S. A new method of inhibiting glycolysis in blood samples. Clin Chim Acta. 1988;172(1):101–8.
Fernandez L, Jee P, Klein MJ, Fischer P, Perkins SL, Brooks SP. A comparison of glucose concentration in paired specimens collected in serum separator and fluoride/potassium oxalate blood collection tubes under survey ‘field’ conditions. Clin Biochem. 2013;46:285–8.
Majkic-Singh N, Sumarac Z, Beletic A. Integrative algorithms in patient focused laboratory medicine. J Med Biochem. 2014;33(1):1.
Simundic AM, Bilic-Zulle L, Nikolac N, Supak-Smolcic V, Honovic L, Avram S, et al. The quality of the extra-analytical phase of laboratory practice in some developing European countries and Mexico—a multicentric study. Clin Chem Lab Med. 2011;49(2):215–28.
Lundberg GD. Acting on significant laboratory results. JAMA. 1981;245(17):1762–3.
Nikolac N, Šupak-Smolčić V, Šimundić AM, Ćelap I. Croatian Society of Medical Biochemistry and Laboratory Medicine: national recommendations for venous blood sampling. Biochem Med. 2013;23(3):242–54.
Küme T, Şişman AR, Özkaya A, Çoker C. Preanalytical errors of specimens sent from the emergency department to the laboratory. Türk Klinik Biyokimya Derg. 2009;7:49–55.
Özcan O, Güreser AS. Sources of preanalytical errors and the role of training in error prevention. Dicle Med J. 2012;39(4):524–30.
Parenmark A, Landberg E. To mix or not to mix venous blood samples collected in vacuum tubes? Clin Chem Lab Med. 2011;49(12):2061–3.
Clinical Laboratory Standards Institute. Procedures for the collection of diagnostic blood specimens by venipuncture. CLSI H3-A6 document. 6th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2007.
CLSI Clinical Laboratory Standards Institute. Procedures for the handling and processing of blood specimens for common laboratory tests. CLSI H18-A4 document. 4th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2010.
Clinical Laboratory Standards Institute. Collection, transport, and processing of blood specimens for testing plasma based coagulation assays and molecular hemostasis assays. CLSI H21-A5 document. 5th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2008.
Melkie M, Girma A, Tsalla T. The practice of venous blood collection among laboratory and non-laboratory professionals working in Ethiopian Government Hospitals: a comparative study. BMC Health Serv Res. 2014;14(1):88.
Del Pino IG, Constanso I, Mourín LV, Safont CB, Vázquez PR. Citric/citrate buffer: an effective antiglycolytic agent. Clin Chem Lab Med. 2013;51(10):1943–9.
Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–72.
Weissman M, Klein B. Evaluation of glucose determinations in untreated serum samples. Clin Chem. 1958;4(5):420–2.
Lin YL, Smith CH, Dietzler DN. Stabilization of blood glucose by cooling with ice: an effective procedure for preservation of samples from adults and newborns. Clin Chem. 1976;22(12):2031–3
Acknowledgements
The authors like to thank—Tamil Nadu State Council of Higher Education—TANSCHE for financial support in the form of M.Sc., “Student Mini Project” to Ms. L. Preethi.
Author information
Authors and Affiliations
Corresponding author
Appendix: Table For Mixing Delay, Centrifugation Delay And Analysing Delay
Appendix: Table For Mixing Delay, Centrifugation Delay And Analysing Delay
Group 1
No samples | No of time inverted | Time taken from completion of centrifuge to analyze | |||||
|---|---|---|---|---|---|---|---|
< 10 min | 30 min | 1.00 h | 1.30 h | 2.00 h | |||
1a, 2a, 3a, 4a, 5a | 2 | Time taken from blood shed to centrifuge Immediately centrifuge (< 10 min) | |||||
1b, 2b, 3b, 4b, 5b | 4 | ||||||
1c, 2c, 3c, 4c, 5c | 6 | ||||||
1d, 2d, 3d, 4d, 5d | 8 | ||||||
1e, 2e, 3e, 4e, 5e | 10 | ||||||
Group 2
No samples | No of time inverted | Time taken from completion of centrifuge to analyze | |||||
|---|---|---|---|---|---|---|---|
< 10 min | 30 min | 1.00 h | 1.30 h | 2.00 h | |||
6a, 7a, 8a, 9a, 10a | 2 | Time taken from blood shed to centrifuge Immediately centrifuge (30 min) | |||||
6b, 7b, 8b, 9b, 10b | 4 | ||||||
6c, 7c, 8c, 9c, 10c | 6 | ||||||
6d, 7d, 8d, 9d, 10d | 8 | ||||||
6e, 7e, 8e, 9e, 10e | 10 | ||||||
Group 3
No samples | No of time inverted | Time taken from completion of centrifuge to analyze | |||||
|---|---|---|---|---|---|---|---|
< 10 min | 30 min | 1.00 h | 1.30 h | 2.00 h | |||
11a, 12a, 13a, 14a, 15a | 2 | Time taken from blood shed to centrifuge (1.00 h) | |||||
11b, 12b, 13b, 14b, 15b | 4 | ||||||
11c, 12c, 13c, 14c, 15c | 6 | ||||||
11d, 12d, 13d, 14d, 15d | 8 | ||||||
11e, 12e, 13e, 14e, 15e | 10 | ||||||
Group 4
No samples | No of time inverted | Time taken from completion of centrifuge to analyze | |||||
|---|---|---|---|---|---|---|---|
< 10 min | 30 min | 1.00 h | 1.30 h | 2.00 h | |||
16a, 17a, 18a, 19a, 20a | 2 | Time taken from blood shed to centrifuge (1.30 h) | |||||
16b, 17b, 18b, 19b, 20b | 4 | ||||||
16c, 17c, 18c, 19c, 20c | 6 | ||||||
16d, 17d, 18d, 19d, 20d | 8 | ||||||
16e, 17e, 18e, 19e, 20e | 10 | ||||||
Group 5
No samples | No of time inverted | Time taken from completion of centrifuge to analyze | |||||
|---|---|---|---|---|---|---|---|
< 10 min | 30 min | 1.00 h | 1.30 h | 2.00 h | |||
21a, 22a, 23a, 24a, 25a | 2 | Time taken from blood shed to centrifuge (2.00 h) | |||||
21b, 22b, 23b, 24b, 25b | 4 | ||||||
21c, 22c, 23c, 24c, 25c | 6 | ||||||
21d, 22d, 23d, 24d, 25d | 8 | ||||||
21e, 22e, 23e, 24e, 25e | 10 | ||||||
Group 6 (EDTA Sample)
No samples | No of time inverted | Time taken from completion of centrifuge to analyze | |||||
|---|---|---|---|---|---|---|---|
< 10 min | 30 min | 1.00 h | 1.30 h | 2.00 h | |||
26a, 27a, 28a, 9a, 30a | 2 | Time taken from blood shed to centrifuge (1 h .30 min) | |||||
31b, 32b, 33b, 34b, 35b | 4 | ||||||
36c, 37c, 38c, 39c, 40c | 6 | ||||||
41d, 42d, 43d, 44d, 45d | 8 | ||||||
46e, 47e, 48e, 49e, 50e | 10 | ||||||
Group 7 (Serum Sample)
No samples | No of time inverted | Time taken from completion of centrifuge to analyze | |||||
|---|---|---|---|---|---|---|---|
< 10 min | 30 min | 1.00 h | 1.30 h | 2.00 h | |||
51a, 52a, 53a, 54a, 55a | 2 | Time taken from blood shed to centrifuge (1 h .30 min) | |||||
56b, 571b, 58b, 59b, 60b | 4 | ||||||
61c, 62c, 63c, 64c, 65c | 6 | ||||||
66d, 67d, 8d, 69d, 70d | 8 | ||||||
71e, 72e, 73e, 74e, 75e | 10 | ||||||
Rights and permissions
About this article
Cite this article
Loganathan, P., Gasper, S.K., Afel, F.K. et al. Pre-analytical Errors in Glucose Estimation Results in Query on Diabetic Management. Ind J Clin Biochem 35, 32–42 (2020). https://doi.org/10.1007/s12291-018-0782-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-018-0782-6