Abstract
Purpose
The mechanism underlying primordial follicle activation is poorly understood. In this study, in-vitro culture and subsequent xenotransplantation were conducted to determine whether testosterone promotes the activation of porcine primordial follicles.
Methods
Prepubertal porcine ovarian cortical strips containing primordial follicles were cultured in the presence of testosterone for 7 days, and subsequently transplanted to immunodeficient mice for 2 months. After culture and transplantation, development of follicles was examined histologically. The presence of androgen receptors in oocytes was assessed by use of western blot and immunohistochemical analyses.
Results
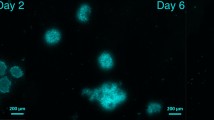
Testosterone at 10−6 M induced the primordial follicle transition to the intermediate (19 ± 4%) and primary (3 ± 1%) stages after 7-day culture, while 56 ± 5% of primordial follicles remained in the initial pool. Higher concentrations, above 10−5 M, or lower concentrations, below 10−6 M, did not induce follicle transition to the primary stage. After 7-day culture with 10−6 M testosterone, ovarian cortical strips were transplanted to immunodeficient mice. Some of the follicles developed to the secondary (15 ± 3%) and antral (10 ± 3%) stages, whereas 44 ± 7% of primordial follicles remained in the initial pool. In the culture experiment, estradiol-17β (10−7–10−5 M) had no significant effect on follicle activation. The androgen receptor antagonist, cyproterone acetate, inhibited the stimulatory effect of testosterone on primordial follicle activation, suggesting an androgen receptor-mediated action of testosterone. Western blot and immunohistochemical analyses revealed that androgen receptors were present in the oocytes of primordial follicles.
Conclusions
These results suggest that testosterone at 10−6 M promotes the activation of porcine primordial follicles in vitro through the androgen receptors in the oocytes.




Similar content being viewed by others
References
Reynaud K, Driancourt MA. Oocyte attrition. Mol Cell Endocrinol. 2000;163:1–8.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–55.
Fortune JE, Cushman RA, Kito WS. The primordial follicle to primary follicle transition. Mol Cell Endocrinol. 2000;163:53–60.
Picton HM. Activation of follicle development: the primordial follicle. Theriogenology. 2001;55:1193–210.
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–80.
Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–15.
Da Silva-Buttkus P, Jayasooriya GS, Mora JM, Mobberley M, Ryder TA, Baithun M, et al. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J Cell Sci. 2008;121:3890–900.
Lintern-Moore S, Moore GP. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod. 1979;20:773–8.
Hirshfield AN. Theca cells may be present at the outset of follicular growth. Biol Reprod. 1991;44:1157–62.
Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207.
Silva JR, van den Hurk R, de Matos MH, dos Santos RR, Pessoa C, de Moraes MO, et al. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology. 2004;61:1691–704.
Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod. 1996;55:942–8.
Fortune JE, Kito S, Wandji SA, Srsen V. Activation of bovine and baboon primordial follicles in vitro. Theriogenology. 1998;49:441–9.
Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–6.
Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12:1993–2001.
Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13:1133–8.
Oktay K, Newton H, Gosden RG. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril. 2000;73:599–603.
Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999;60:1462–7.
Candy CJ, Wood MJ, Whittingham DG. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum Reprod. 1995;10:2334–8.
Gosden RG, Boulton MI, Grant K, Webb R. Follicular development from ovarian xenografts in SCID mice. J Reprod Fertil. 1994;101:619–23.
Kaneko H, Kikuchi K, Noguchi J, Hosoe M, Akita T. Maturation and fertilization of porcine oocytes from primordial follicles by a combination of xenografting and in vitro culture. Biol Reprod. 2003;69:1488–93.
Senbon S, Ota A, Tachibana M, Miyano T. Bovine oocytes in secondary follicles grow and acquire meiotic competence in severe combined immunodeficient mice. Zygote. 2003;11:139–49.
Moniruzzaman M, Miyano T. KIT-KIT ligand in growth of porcine primordial follicles. J Reprod Dev. 2007;53:1273–81.
Moniruzzaman M, Lee J, Zengyo M, Miyano T. Knockdown of FOXO3 induces primordial oocyte activation in pigs. Reproduction. 2010;139:349–57.
Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol. 2006;4:16.
McNatty KP. Hormonal correlates of follicular development in the human ovary. Aust J Biol Sci. 1981;34:249–68.
Eiler H, Nalbandov AV. Sex steroids in follicular fluid and blood plasma during the estrous cycle of pigs. Endocrinology. 1977;100:331–8.
Chang SC, Jones JD, Ellefson RD, Ryan RJ. The porcine ovarian follicle: 1. Selected chemical analysis of follicular fluid at different developmental stages. Biol Reprod. 1976;15:321–8.
Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–9.
Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–74.
Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod. 2006;75:924–32.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Burek M, Duda M, Knapczyk K, Koziorowski M, Słomczyńska M. Tissue-specific distribution of the androgen receptor (AR) in the porcine fetus. Acta Histochem. 2007;109:358–65.
Takeda H, Chodak G, Mutchnik S, Nakamoto T, Chang C. Immunohistochemical localization of androgen receptors with mono- and polyclonal antibodies to androgen receptor. J Endocrinol. 1990;126:17–25.
O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–6.
Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. J Reprod Fertil. 1994;100:333–9.
Miyano T, Hirao Y. In vitro growth of oocytes from domestic species. J Mam Ova Res. 2003;20:78–85.
Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL, et al. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology. 2010;151:774–82.
Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80:726–36.
Ainsworth L, Tsang BK, Downey BR, Marcus GJ, Armstrong DT. Interrelationships between follicular fluid steroid levels, gonadotropic stimuli, and oocyte maturation during preovulatory development of porcine follicles. Biol Reprod. 1980;23:621–7.
Cheng G, Weihua Z, Mäkinen S, Mäkelä S, Saji S, Warner M, Gustafsson JA, Hovatta O. A role for the androgen receptor in follicular atresia of estrogen receptor beta knockout mouse ovary. Biol Reprod. 2002;66:77–84.
Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005;19:1792–802.
Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004;71:1712–23.
Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321.
Słomczyñska M, Tabarowski Z. Localization of androgen receptor and cytochrome P450 aromatase in the follicle and corpus luteum of the porcine ovary. Anim Reprod Sci. 2001;65:127–34.
Juengel JL, Heath DA, Quirke LD, McNatty KP. Oestrogen receptor alpha and beta, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction. 2006;131:81–92.
Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Müllerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1034–40.
Acknowledgments
We are grateful to the staff of the Kobe Meat Inspection Office for supplying pig ovaries. This study was supported in part by the Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to TM and MM.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Magamage, M.P.S., Zengyo, M., Moniruzzaman, M. et al. Testosterone induces activation of porcine primordial follicles in vitro. Reprod Med Biol 10, 21–30 (2011). https://doi.org/10.1007/s12522-010-0068-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12522-010-0068-z