Abstract
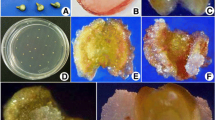
Two media, Gamborg’s medium (B5) and Murashige and Skoog’s medium (MS) with various combinations, were used to study their effect on haploid induction efficiency from unpollinated flowers or ovaries in tropical short day onion. In B5 medium, highest induction efficiency (1.9%) was observed on HAP18 followed by HAP17 and HAP05 whereas in MS medium, HAP40 recorded highest induction frequency (5.0%) followed by HAP32. Kinetin, meta-topolin, thidiazuron did not influence embryo induction. Sucrose at 7.5% in B5 medium and at 10% in MS medium was found to be optimum for induction. Days for plantlet induction were 98.9 ± 3.9 days in B5 medium and 83.1 ± 2.7 days in MS medium. Induction rate of 0.51% and survival of 27.8% in B5 medium and induction rate of 0.72% and survival of 33% in MS medium were observed. Three haploids, 1 mixoploid and 1 diploid in B5 medium and 2 haploids, one diploid in MS medium were obtained. Flow cytometry and cytology confirmed the status of haploid plants. MS medium was found to be better than B5 medium and newer combinations for haploid induction were identified. Plant Preservative Mixture (PPM™) at 1 mg/l was found to be efficient for inhibiting contamination from field grown flower buds. These combinations will be helpful in devising new strategies for faster and higher rate of haploid induction in tropical short day Indian cultivars.





Similar content being viewed by others
References
FAOSTAT (2014) Food and Agriculture Organization of the United Nations—FAO Statistical Database. http://www.fao.org/faostat/en/#data/QC. Assessed 3 June 2017
Singh RK, Bhonde SR (2011) Performance studies of exotic onion (Allium cepa L.) hybrids in the Nashik region of Maharashtra. Indian J Hill Farming 24:29–31
Nunes RLC, Oliveira ABD, Dutra AS (2014) Agronomic performance of onion hybrids in Baraúna, in the semi-arid region of Brazil. Rev Ciênc Agron 45:606–611
Shigyo M, Kik C (2008) Onion. In: Prohens J, Nuez JF (eds) Vegetables II. Springer, New York, pp 121–159
Muren RC (1989) Haploid plant induction from unpollinated ovaries in onion. HortScience 24:833–834
Bohanec B, Jakše M, Ihan A, Javornik B (1995) Studies of gynogenesis in onion (Allium cepa L.): induction procedures and genetic analysis of regenerants. Plant Sci 104:215–224
Alan AR, Brants A, Cobb E, Goldschmied P, Mutschler MA, Earle ED (2004) Fecund gynogenic lines from onion (Allium cepa L.) breeding materials. Plant Sci 167:1055–1066
Fayos O, Vallés MP, Garcés-Claver A, Mallor C, Castillo AM (2015) Doubled haploid production from Spanish onion (Allium cepa L.) germplasm: embryogenesis induction, plant regeneration and chromosome doubling. Front Plant Sci 6:384
Geoffriau E, Kahane R, Rancillac M (1997) Variation of gynogenesis ability in onion (Allium cepa L.). Euphytica 94:3–44
Jakše M, Hirschegger P, Bohanec B, Havey MJ (2010) Evaluation of gynogenic responsiveness and pollen viability of selfed doubled haploid onion lines and chromosome doubling via somatic regeneration. J Am Soc Hortic Sci 135:67–73
Bohanec B, Jakše M (1999) Variations in gynogenic response among long-day onion (Allium cepa L.) accessions. Plant Cell Rep 18:737–742
Dunstan DI, Short KC (1977) Improved growth of tissue cultures of the onion, Allium cepa. Physiol Plant 41:70–72
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Michalik B, Adamus A, Nowak E (2000) Gynogenesis in Polish onion cultivars. J Plant Physiol 156:211–216
Alan AR, Mutschler MA, Brants A, Cobb E, Earle ED (2003) Production of gynogenic plants from hybrids of Allium cepa L. and A. roylei Stearn. Plant Sci 165:1201–1211
Anandhan S, Chavan AA, Gopal J, Mote SR, Shelke PV, Lawande KE (2014) Variation in gynogenic potential for haploid induction in Indian short-day onions. Indian J Genet 74:526–528
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Niedz RP, Bausher MG (2002) Control of in vitro contamination of explants from greenhouse- and field-grown trees. In Vitro Cell Dev Biol Plant 38:468–471
Miyazaki JJ, Tan BH, Errington SG (2010) Eradication of endophytic bacteria via treatment for axillary buds of Petunia hybrida using Plant Preservative Mixture (PPM™). Plant Cell Tissue Organ Cult 102:365–372
Chiancone B, Karasawa MMG, Gianguzzi V, Abdelgalel AM, Bárány I, Testillano PS, Marinoni DT, Botta R, Germanà MA (2015) Early embryo achievement through isolated microspore culture in Citrus clementina Hort. Ex Tan., cvs. ‘Monreal Rosso’ and ‘Nules’. Front Plant Sci 6:413
Chukwujekwu JC, Fennell CW, Van Staden J (2002) Optimisation of the tissue culture protocol for the endangered Aloe polyphylla. S Afr J Bot 68:424–429
Chen UC, Hsia CN, Yeh MS, Agrawal DC, Tsay HS (2006) In vitro micropropagation and ex vitro acclimation of Bupleurum kaoi—an endangered medicinal plant native to Taiwan. In Vitro Cell Dev Biol Plant 42:128–133
Sulistyaningsih EYA, Tashiro Y (2006) Flower bud culture of shallot (Allium cepa L. Aggregatum group) with cytogenetic analysis of resulting gynogenic plants and somaclones. Plant Cell Tissue Organ Cult 86:249–255
Jakse M, Havey MJ, Bohanec B (2003) Chromosome doubling procedures of onion (Allium cepa L.) gynogenic embryos. Plant Cell Rep 21:905–910
Campion B, Alloni C (1990) Induction of haploid plants in onion (Allium cepa L.) by in vitro culture of unpollinated ovules. Plant Cell Tissue Organ Cult 20:1–6
Campion B, Azzimonti MT, Vicini E, Schiavi M, Falavigna A (1992) Advances in haploid plant induction in onion (Allium cepa L.) through in vitro gynogenesis. Plant Sci 86:97–104
Jakše M, Bohanec B, Ihan A (1996) Effect of media components on the gynogenic regeneration of onion (Allium cepa L.) cultivars and analysis of regenerants. Plant Cell Rep 15:934–938
Bohanec B, Jakše M, Havey MJ (2003) Genetic analyses of gynogenetic haploid production in onion. J Am Soc Hortic Sci 128:571–574
Acknowledgements
This study was supported by the Department of Biotechnology, Government of India under the project code BT/PR12181/BPA/118/22/2014. The authors are thankful to the Director, ICAR-IARI and Head, Division of Vegetable Science, ICAR-IARI for providing the laboratory facilities and encouragement to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Significance statement India is the second largest producer of onion but the productivity is very low. Low productivity is because of lack of hybrids and farmers grow open pollinated varieties. For hybrid development, inbreds are a prerequisite and for inbred development through conventional methods 12–14 years are required. Hence, hybrids have not been released in India till date. Haploid culture will help in generating inbreds in 2 years, instead of 12–14 years, which will accelerate hybrid onion development.
Rights and permissions
About this article
Cite this article
Mathapati, G.B., Kalia, P., Islam, S. et al. Influence of Culture Media and Their Compositions on Haploid Induction in Indian Short Day Onion. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 739–746 (2019). https://doi.org/10.1007/s40011-018-0990-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-018-0990-0