Abstract
Human amnion–derived mesenchymal stem cell (hAD-MSC) transplantation can repair ovarian injury and improve ovarian function in rats with chemotherapy-induced primary ovarian insufficiency (POI). However, ensuring that stem cells home to the ovary to improve their effects on ovarian injury is challenging. This research aimed to directly inject ovarian tissue with hAD-MSCs and improve the homing of stem cells to the ovary. The animals were divided into POI, hAD-MSC (tail vein) treatment, hAD-MSC (in situ) treatment, and control groups. POI rat models were established by intraperitoneal injection of cyclophosphamide (CTX) and busulfan (BUS). The hAD-MSCs isolated from the amnion were injected into the tail vein or ovary of POI rats. The estrous cycle, serum sex hormone levels, follicle counts, ovarian pathological changes, and proteome of the ovaries were evaluated. hAD-MSCs were successfully isolated and cultured from the amnion. Both hAD-MSC (tail vein) and hAD-MSC (in situ) transplantation increased body weight, improved the AMH levels and follicle numbers, and reduced reproductive organ injuries in POI rats. Transplantation of hAD-MSCs (in situ) upregulated 24 proteins and downregulated 4 proteins. Both hAD-MSC (tail vein) and hAD-MSC (in situ) transplantations can repair ovarian injury and improve ovarian function in rats with chemotherapy-induced POI. The paracrine proteome of hAD-MSCs in the ovarian microenvironment can protect against chemotherapy-induced damage by reducing apoptosis and promoting angiogenesis, cell proliferation, and gene expression.
Similar content being viewed by others
Introduction
In 2018, 10,590 children (from birth to 14 years of age) were estimated to have been diagnosed with cancer (excluding benign/borderline malignant brain tumors) in the USA [1]. In particular, the use of multiagent chemotherapy has increased the combined 5-year survival rate for childhood cancers in Europe to ~ 70–82% [2]. This translates into ~ 300,000 and 500,000 childhood and adolescent cancer survivors in Europe, respectively [3]. Chemotherapy, especially exposure to high doses of alkylating agents, is a well-known risk factor for primary ovarian insufficiency (POI), acute ovarian failure, and reduced fertility due to premature depletion of the follicular pool [4, 5]. POI seriously affects a woman’s health, causing infertility, menopausal symptoms, cardiovascular issues, decreased bone density, reduced psychological function, and other problems [6,7,8], indicating that studies and treatment of POI are of great importance. Recently, researchers have shown an increased interest in the intravenous injection of mesenchymal stem cells to cure POI. Intravenous injection of stem cells can improve damaged ovarian function, but how the cells home to damaged ovarian tissue to play a better role in repairing the injury is a challenge. A previous study showed that human amnion–derived mesenchymal stem cell (hAD-MSC) transplantation can repair ovarian injury and improve ovarian function in rats with chemotherapy-induced POI [9]. This research aimed to directly inject ovarian tissue with hAD-MSCs and improve the homing of stem cells to the ovary.
Materials and Methods
Isolation and Culture of hAD-MSCs
This research was in accordance with the Helsinki Declaration and approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. Primary hAD-MSCs were isolated, cultured, and identified according to our previously published protocols [9]. The third to fifth passages of hAD-MSCs were used for subsequent experiments.
Labeling and Tracking of hAD-MSCs
To track and locate the transplanted hAD-MSCs in the tissues of rats, the hAD-MSCs were labeled with the PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich, St. Louis, MO, USA). The PKH26-labeled hAD-MSCs were transplanted into rats via the tail vein and by ovarian in situ injection (hAD-MSCs were injected directly into the ovaries). At the fourth week after cell transplantation, ovaries and other organs were fixed with optimal cutting temperature (OCT) compound and cut into fresh sections (10 μm thick). After fixation with precooled acetone for 10 min, the sections were washed and incubated with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; Boster Biological Technology Co. Ltd., Wuhan, Hubei, China) at room temperature for 10 min. The sections were subsequently imaged under a fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Animal Model Establishment and Grouping
In total, 48 female Sprague-Dawley (SD) rats (8–10 weeks of age) were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). Animal experimental protocols were approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. These animals were randomly divided into control, POI, hAD-MSC treatment (tail vein), and hAD-MSC treatment (in situ) groups (n = 12 each group). To establish the POI model [10], the rats from the POI, hAD-MSC (tail vein), and hAD-MSC (in situ) groups were treated once by intraperitoneal injection (IP) with cyclophosphamide (CTX, Shengdi Pharmaceutical Co., Ltd., China) dissolved in 0.9% saline solution at a dose of 83.52 m/kg (according to the rat and mouse drug dose conversion [11], 120 × 0.696) combined with busulfan (BUS,C6H14O6S2, purity 99%, Shanghai Aladdin Technology Co., Ltd., China) mixed with sesame oil (Acros Co., Ltd., USA) at a dose of 20.88 mg/kg (according to the drug dose conversion [11], 30 × 0.696). The rats from the control group were injected with normal saline and sesame oil. At 24 h after chemotherapy, the rats from the hAD-MSCs (tail vein) group were injected with 0.6 ml of phosphate-buffered saline (PBS) containing 4 × 106 hAD-MSCs labeled with PKH-26 via the tail vein and 0.025 ml PBS by ovarian in situ injection. The rats from the hAD-MSC (in situ) group were injected with 0.6 ml of PBS via the tail vein and 0.050 ml of PBS containing 4 × 106 hAD-MSCs labeled with PKH-26by ovarian in situ injection. The POI and control groups were injected with 0.6 ml of PBS via the tail vein and 0.050 ml of PBS by ovarian in situ injection. At 1 week after the CTX and BUS injection, the vaginal smears of twelve rats from each group were obtained at 9.00 am daily for 4 weeks to examine the estrous cycle [12]. The rats in the stages of estrus, proestrus, metestrus, and diestrus were identified and quantified by vaginal smears. On the first day of chemotherapy, the animals in the four groups were weighed every week for 4 consecutive weeks. Four weeks after transplantation, the rats were narcotized, and the ovaries and blood were collected. Some ovaries were fixed with 4% paraformaldehyde, and the others were frozen in a liquid nitrogen tank or with OCT compound (Sakura Finetek USA Inc., Torrance, CA, USA) for further processing. Serum was collected by centrifugation and stored at − 80 °C for hormone tests.
Enzyme-Linked Immunosorbent Assay
Whole-blood samples (3.0–5.0 mL) of 10 rats in each group were harvested by heart puncture after the rats were anesthetized with pentobarbital sodium at a dose of 40 mg/kg by IP 28 days, after the first dose of drug use. Then, the rats were sacrificed. The blood sample was centrifuged at 4000 rpm for 5 min at room temperature and the supernatants were harvested. The serum levels of anti-Müllerian hormone (AMH), FSH, and estradiol (E2) were measured as indicators to assess sex hormone levels in rat models using an enzyme-linked immunosorbent assay kit (Wuhan Colorful-Gene biological technology Co., Ltd., China).
Pathological Examination
After the rats were sacrificed, ovaries were also subjected to histological analysis. The harvested organs were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned to a 5-μm thickness, and stained with hematoxylin and eosin (H&E). The follicles in the ovarian sections were examined, and the damage to the follicles was evaluated. Follicles were classified as follows and counted in every fifth section of the ovary [13]: primordial follicle (oocyte surrounded by several granulosa cells with flattened nuclei), primary follicle (oocyte surrounded by one layer of granulosa cells with cuboidal cells), secondary follicle (oocyte surrounded by more than one layer of granulosa cells with cuboidal cells) [14], and atretic follicle (broken-down form of the ovarian follicles, which consists of an oocyte surrounded by granulosa cells and internal and external theca cells [15]).
Proteome Profiler
At the fourth week after cell transplantation, ovaries were collected from the hAD-MSC (in situ) and POI groups. The protein concentration was measured as indicators in the proteome profiler assay kit (R&D; Cat ARY002B and R&D; Cat ARY015, R&D Systems, Inc., Ltd., USA). Each membrane of the protein chip needed to be closed with 2 ml of the corresponding array buffer and incubated for 1 h on a shaker. The sample should be diluted with the corresponding array buffer to a volume of 1.5 ml per well and incubated with the diluted detection antibody for 1 h at room temperature. Then, the corresponding array unit was added and incubated with the array protein membrane overnight at 4°. The membrane was washed three times with the corresponding wash buffer, streptavidin-HRP was added, the membrane was incubated for 30 min, and the membrane was shaken in the shaker at room temperature. The membrane was washed three times with the corresponding wash buffer, 1 ml of chemical color reagent was added to each well, and the color was developed on a chemiluminescence imager. A relative change ratio in protein expression in the hAD-MSC group of ≥ 0.2 or ≤ − 0.2 was considered significantly different and was considered to be a more important protein candidate involved in hAD-MSC-mediated effects.
Western Blotting
Tissue proteins were extracted according to the manufacturer’s instructions. Protein samples (25–50 μg total protein per lane) were resolved by SDS-PAGE and then transferred to a PVDF membrane (Millipore, USA). After the membrane was blocked with 5% skim milk powder, it was incubated with specific antibodies overnight at 4 °C and then with a suitable horseradish peroxidase–conjugated secondary antibody (Abbkine, USA). Protein bands were visualized using the Western Bright TM ECL System (APG Bio, China). Antibodies were against JNK2 (Abcam, USA), p38 (Abcam, USA), serpin E1 (Abcam, USA), and GDPH (Abcam, USA).
Statistical Analysis
Data are expressed as the mean ± SD. Statistical analysis was performed with SPSS 22.0 software (IBM, Armonk, NY, USA). The independent-samples t test and one-way analysis of variance (ANOVA) were used for two- and multiple-group comparisons, respectively. Statistical significance was set at P < 0.05.
Results
In Vivo Tracking of hAD-MSCs
To track and locate the hAD-MSCs in vivo, the cells were prelabeled with PKH26 before transplantation. As detected by flow cytometry, the cell labeling rate was 99.47% (Fig. 1d). The location and fate of the transplanted PKH26-labeled hAD-MSCs in the ovarian tissue were tested at 4 weeks after cell transplantation (Fig. 1f). The results showed that the PKH26-labeled cells were only located in the interstitial of the ovaries, rather than in the follicles, after transplantation in both the hAD-MSC (tail vein) and hAD-MSC (in situ) groups. The fluorescence signal distribution in the ovarian tissue of the hAD-MSC (in situ) group was stronger than that in the ovarian tissue of the hAD-MSC (tail vein) group, and the E2 level was significantly higher in the hAD-MSC (in situ) group than in the model group.
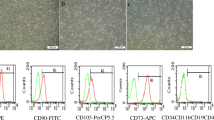
Morphology and multipotency of hAD-MSCs. a, b, c Cell morphology of passage 1 (a), 3 (b), and 5 (c) hAD-MSCs (× 100). hAD-MSCs tracked in vivo. The labeling rates of PKH26-labeled hAD-MSCs (d, labeled, e unlabeled) were detected by flow cytometry. f Transplanted hAD-MSCs were observed at 4 weeks after cell transplantation in ovaries (× 100). hAD-MSCs, human amnion–derived mesenchymal stem cells
Transplantation of hAD-MSCs Increases the Body Weights of POI Rats
The body weights of the rats were measured next. Our results showed that the body weights of the rats in the hAD-MSC (tail vein) and hAD-MSC (in situ) groups were significantly increased after chemotherapy compared with that of the POI group at the fourth week after cell transplantation (P < 0.05; Fig. 2d). Moreover, compared with that of the control group, the body weights in the POI groups were significantly decreased starting from the third week (P < 0.05; Fig. 2d). However, there was no significant difference between the hAD-MSC (tail vein) and hAD-MSC (in situ) groups. These results demonstrate that both hAD-MSC (tail vein) and hAD-MSC (in situ) transplantation can increase the body weights of POI rats.
Transplantation of hAD-MSCs improves ovarian function in POI rats. a Vaginal smears were obtained and the estrous cycles were evaluated by H&E staining in the second and fourth week. Representative photographs for estrus, proestrus, metestrus, and diestrus are shown (× 100). b The percentage of rats with normal cyclicity was determined at 2 and 4 weeks after cell transplantation. c hAD-MSCs were injected into the ovaries. d Changes in the body weights of SD rats after transplantation with hAD-MSCs. e, f, g Serum levels of AMH (e), FSH (f), and E2 (g) were measured at 4 weeks after cell transplantation. Scale bars = 100 μm. *P < 0.05, **P < 0.01. AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; hAD-MSCs, human amnion–derived mesenchymal stem cells; POI, primary ovarian insufficiency
Transplantation of hAD-MSCs Improves Ovarian Function in POI Rats
To investigate the effects of hAD-MSC (tail vein) and hAD-MSC (in situ) transplantation on ovarian function in POI rats, the estrous cycle and serum levels of AMH, E2, and FSH were examined. The results showed that 16.7% of the rats had irregular estrous cycles in the second week after chemotherapy. At the second and fourth weeks after hAD-MSC transplantation, vaginal smears from the four groups were examined (Fig. 2a, b). The results showed that the percentages of rats with abnormal cyclicity are decreased in both the hAD-MSC (tail vein) and hAD-MSC (in situ) groups from the second week after cell transplantation but had an increasing trend compared with that in the POI group, although there were no significant differences in the cyclicity of estrus in the POI and hAD-MSC groups. On the other hand, compared with the POI group, the levels of AMH (indicating ovarian reserve) were significantly increased in the hAD-MSC (tail vein) and hAD-MSC (in situ) groups at the fourth week after cell transplantation (P < 0.05; Fig. 2e). Moreover, compared with that of the POI group, the FSH level was significantly decreased in the hAD-MSC (tail vein) and hAD-MSC (in situ) groups at the fourth week after cell transplantation (Fig. 2f), while the E2 level was significantly increased in the hAD-MSCs (in situ) group at the fourth week after cell transplantation (Fig. 2g). However, there was no significant difference between the hAD-MSC (tail vein) and hAD-MSC (in situ) groups. These results demonstrate that both hAD-MSC (tail vein) and hAD-MSC (in situ) transplantation can protect the ovarian reserve and improve ovarian function in POI rats.
Transplantation of hAD-MSCs Reduces Reproductive Organ Injuries in POI Rats
Ovaries from the four groups were collected and subjected to pathological analysis. Our results showed that there were significant differences in primordial, primary, secondary, and mature follicles in the POI group compared with the control group, while there were no significant differences in atretic follicles in the POI group compared with the control group (P < 0.05; Fig. 3a, b). Moreover, compared with the POI group, the numbers of follicles at the various stages were significantly increased in both the hAD-MSC (tail vein) and hAD-MSC (in situ) groups in the fourth week after cell transplantation (P < 0.05; Fig. 3b), while the number of atretic follicles was not significantly decreased in the hAD-MSC (tail vein) and hAD-MSC (in situ) groups. These changes were improved by cell transplantation in the hAD-MSC (tail vein) and hAD-MSC (in situ) groups. However, there was no significant difference between these two groups. Graft-versus-host disease was not found in any transplanted rats, and no obvious organic lesions were observed in the livers, hearts, lungs, spleens, or kidneys after chemotherapy. Taken together, these results demonstrate that both hAD-MSC (tail vein) and hAD-MSC (in situ) transplantation can reduce ovarian injury in POI rats.
Transplantation of hAD-MSCs reduces reproductive ovarian injuries in POI rats. a Pathological changes in the ovaries were evaluated by H&E staining in the four groups at 4 weeks after hAD-MSC transplantation (× 100). b The number of follicles at various stages was counted and compared between the four groups. Scale bars = 100 μm. *P < 0.05, **P < 0.01. hAD-MSCs, human amnion–derived mesenchymal stem cells; POI, primary ovarian insufficiency
Use of the Proteome Profiler Arrays as a Screening Tool to Identify the Protein of Interest
To better understand the paracrine proteome of hAD-MSCs, we evaluated components in ovarian tissue after hAD-MSC injection using proteome profiler arrays that examined 74 human cytokines (the ovaries of the POI group were used as the control). Then, we measured 74 cytokines and ranked them according to the value of the hAD-MSC group minus that of the POI group by pixel density from highest to lowest. We classified these 74 cytokines into two groups that are important in the hAD-MSC-mediated recovery of ovarian function, namely, regulation of MAPK signaling (21 proteins) and angiogenesis (53 proteins) (Fig. 4a). There were 24 upregulated proteins and 4 downregulated proteins. It is suggested that hAD-MSCs may improve the function of the ovary in POI rats by regulating these proteins.
Use of proteome profiler arrays as a screening tool to identify proteins of interest. Protein extract (400 μg) was used for array analysis. Array spots were visualized according to the manufacturer’s instructions. The figure shows significant differences in the relative changes in protein expression during hAD-MSC treatment. The figure shows the relative changes in protein values. The relative changes in protein expression in the hAD-MSC group ≥ 0.2 or ≤ − 0.2 are significantly different, and these proteins are considered to be the important protein candidates that are involved in mediating hAD-MSC effects. Upregulated antibody (a phospho-mitogen-activated kinase (MAPK) array, b angiogenesis array). The data are shown as the average of two separate samples. hAD-MSCs, human amnion–derived mesenchymal stem cells; POI, primary ovarian insufficiency
Western Blot Verification of the Partial Protein Results of the Proteome Profiler Arrays
Western blot analysis showed that the protein expression of JNK2, p38, and serpin E1 was significantly increased after transplantation with hAD-MSCs (in situ), and the difference was statistically significant (Fig. 5). The expression of JNK2 was also promoted after hAD-MSC injection in the tail vein. The difference was statistically significant. Although there was no significant difference in p38 and serpin E1 expression, there were increasing trends.
Discussion
The present study was designed to determine the effect of hAD-MSCs injected into the ovary on POI in SD rats. POI is a clinical syndrome defined as the loss of ovarian activity before the age of 40 years and is characterized by menstrual disturbance (amenorrhea or oligomenorrhea) with increased levels of gonadotropin and decreased levels of estradiol [16]. In recent years, many researchers have focused on POI treatment, including ovarian tissue cryopreservation, stem cell therapy, hormone replacement therapy, and fertility preservation programs [17,18,19,20] as well as the preservation of ovarian function and fertility [21]. Most studies in the field of stem cell therapy have focused on tail vein injection [13, 22].These methods are unsatisfactory because it is difficult for stem cells to reach the ovaries. This study aimed to overcome the bottleneck regarding the homing of stem cells to the ovary. HAD-MSCs are derived from the human placental amnion and involve no damage, waste recycling, extensive materials, or ethical conflicts. hAD-MSC transplantation can repair ovarian injury and improve ovarian function in rats with chemotherapy-induced POI at least partly through a paracrine mechanism [9, 23]. Therefore, this study used hAD-MSC injection into the ovary to promote the homing of hAD-MSCs, so that hAD-MSCs could better exert their effects in terms of alleviating POI. This method is promising in its ability to better provide better raw materials for tissue engineering in the future and improve the health of patients with POI.
A large number of hAD-MSCs can be isolated from human term amnion, and hAD-MSCs proliferate quickly in vitro. The xenotransplantation of hAD-MSCs does not induce xenogeneic immune responses in rats with POI, suggesting the low immunogenicity of hAD-MSCs [9, 24]. The results of this study indicate that hAD-MSCs can be differentiated into three germ layers by in vitro culture. However, the marker, which was traced in the animal, was mainly distributed around the follicle and was not found to differentiate into granulosa cells or oocytes, which is similar to other report [25]. hAD-MSCs improve ovarian function through a paracrine mechanism rather than directed transformation into germ cells. Moreover, the distribution of labeled cells to the ovary has a tendency to be higher than that with the tail vein injection, which suggests that local injection of cells may allow more cells to colonize the ovarian tissue. It is better to perform quantitative and stereological studies with fluorescence.
Very little was found in the literature regarding hAD-MSCs by ovarian in situ injection. The most notable finding to emerge from the analysis was hAD-MSCs transplantation dramatically improved the estrus cycle rate, reduced weight loss and FSH levels, increased AMH and E2 levels, reduced the loss of primordial follicles, primary follicles, second follicles, mature follicles, and repaired ovarian injury and improved ovarian function. Compared with hAD-MSC (tail vein), there was no statistically significant difference in hAD-MSC (in situ), but there was a trend toward increasing body weight, improving AMH levels, and increasing E2 levels. Previous studies have shown that MSCs can inhibit cell apoptosis and enhance cell survival through paracrine pathways [9], which thus exert positive effects on repairing ovarian tissue damage. However, the observed difference between the experiment and control group in this study was not significant. A possible explanation for these results may be the lack of an adequate number of follicles in the POI group, while the control group had a large enough number of follicles and decay at a normal rate.
Alkylating agents such as cyclophosphamide and busulfan have high ovarian toxicity and can cause the destruction, depletion, vascular damage, and cortical fibrosis of ovaries. A previous study showed that 42% of women treated with alkylating agents developed POI [26]. Cyclophosphamide induces direct activation of primordial follicles, which grow to a stage that responds to FSH via PTEN inhibition, thus triggering the accelerated depletion of primordial follicles to induce POI.
Ovarian germ stem cells are derived from potential stem cells in the cortex, which can produce new stem cells by symmetric division, or differentiate into germ cells and primitive granulosa cells by asymmetric division [27, 28]. Previous studies have demonstrated the existence of ovarian germ stem cells, which can differentiate into reproductive cells and primitive GCs to replenish the follicle pool [27, 29]. The ovarian germ stem cell niche, also called the stem cell microenvironment, plays an important role in ovarian repair after injury. The germ stem cell niche is composed of extracellular matrix, niche cells, and cytokines surrounding the ovarian germ stem cells [28, 30]. Studies have shown that ovarian insufficiency is mainly due to the aging of the ovarian germ stem cell niches but not due to the aging of the ovarian germ stem cells [27, 28, 31, 32]. Some researchers have successfully isolated ovarian germ stem cells from the ovaries of mice and humans with POI33, and ovarian germ stem cells from POI ovaries have the ability to differentiate into oocytes in vitro [33]. Thus, the microenvironment of ovarian tissue plays an important role in mediating ovarian function and aging [28], and improving the microenvironment of ovaries in POI might cause the ovarian germ stem cells to remain active and continue to supplement the follicle pool. Winkler et al. found that CTX injured the stem cell niche [34]. Sun et al. found that transplantation of BM-MSCs was effective in inducing osteoblastic niche reconstruction and improving the hematopoietic stem cell niche [35].One can hypothesize that these conditions are likely to occur in the ovary after injection with hAD-MSCs. Additionally, it has been reported that MSC transplantation reduces the depletion of germ stem cells induced by CTX [36]. Lai et al. also showed that MSC transplantation further reduced the depletion of follicles at various stages after chemotherapy, including primordial follicles [36]. In this study, we found that the number of follicles increased in both the hAD-MSC (tail vein) and hAD-MSC (in situ) groups. However, there was no significant difference between those two groups. Further research is needed to investigate the mechanism underlying the effects of hAD-MSCs on follicles.
Regarding the molecular mechanism through which hAD-MSCs improve ovarian function in POI, this study found that upregulated proteins included Akt (protein kinase B), MKK3/6 (MAPK (mitogen-activated protein kinase) kinase3/6), RSK1 (ribosomal S6 kinases 1), p38β, JNK2 (c-Jun N-terminal kinase 2), MMP, and serpin E1. Akt regulates many processes including metabolism, proliferation, cell survival, growth, and angiogenesis. Low levels of p-Akt inhibited meiosis in oocytes, increases serum follicle-stimulating hormone levels, regulates early folliculogenesis by maintaining the dormancy of primordial follicles, decreases ovary size, and reduces follicle numbers, consistent with POI [37,38,39]. The PI3K/Akt (phosphatidylinositol-3-kinase/protein kinase B) signaling pathway participates in the recovery of ovarian function by changing the ratios of Th17/ Tc17 and Th17/Treg cells in POF mice following MSC transplantation [40]. MSCs participate in the activation of ovarian transcriptional expression in the ECM-dependent AKT signaling pathway and thus restore ovarian function to a certain extent by reducing apoptosis and promoting angiogenesis [41] (Fig. 6a). In murine and human ovaries, phosphatase and tensin homolog inhibitors and phosphatidylinositol-3-kinase activators have been demonstrated to activate dormant primordial follicles in in vitro cultures [42]. JNK2 coordinate cell responses to stress and influences the regulation of cell growth and transformation. Overexpression of JNK represents a novel mechanism for regulating meiotic initiation and is related to reducing POI in humans [43]. In the JNK pathway, activation of JNK after activation of MEKK also activates p38, phosphorylating the transcription factors Elk-1 and ATF2, thereby enhancing transcriptional activity. MKK3/6 is located upstream of the MAP kinases and is responsible for their activation. RSK1 is an intracellular serine/threonine kinase that is an important signaling intermediate in response to a broad range of ligand-activated receptor tyrosine kinases. p38 is one of the subtypes of MAPKs, and its nature is similar to that of JNK. Upstream MKK3 and MKK6 can specifically activate p38. After activation, p38 MAPK becomes an active protease, which is transferred into the nucleus to activate many transcription factors through phosphorylation, thereby regulating gene expression. Activation of the MAPK signaling pathway initiates downstream ERK1/2 kinase and is involved in the regulation of cellular life activities, such as protein synthesis, gene expression, and cell differentiation (Fig. 6b). Cell-reactive cytokines such as TNF-a, IL-1, and IL-6 are expressed. Matrix metalloproteinases (MMPs) allow cell- and follicle-driven remodeling because the abundance of collagen in natural ovarian tissues undergoes remodeling during follicular development and ovulation [44].
Schematic diagram illustrating the possible mechanisms responsible for the restorative effects of hAD-MSC-based therapy. a The paracrine proteome of hAD-MSCs functions in the ovarian microenvironment of a chemotherapy-induced POI model by reducing apoptosis and promoting angiogenesis. b The paracrine proteome of regular MAPK signaling and angiogenic signal function as in the ovarian microenvironment with chemotherapy-induced damage via cell proliferation and gene expression
Conclusion
Regarding the question posed at the beginning of this study, it is now possible to state that hAD-MSCs have multiple differentiation potentials. Both hAD-MSC (tail vein) and hAD-MSC (in situ) transplantation can reduce ovarian injury and enhance ovarian function in SD rats with chemotherapy-induced POI. hAD-MSCs injected into the ovary are more capable of reducing inflammation triggered by chemotherapy in ovarian tissue. hAD-MSCs can regulate cytokines through the MAPK and angiogenesis pathways. These findings raise important theoretical issues that affect the paracrine pathway, contribute in several ways to our understanding of the hAD-MSC secretome, and provide a basis for further research. One source of weakness in this study that may have affected the measurements of PKH26-labeled hAD-MSCs is the lack quantification of tissue fluorescence.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on a reasonable request.
Abbreviations
- POI:
-
primary ovarian insufficiency
- CTX:
-
cyclophosphamide
- BUS:
-
busulfan
- CTX:
-
cyclophosphamide
- DAPI:
-
2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride
- EDTA:
-
ethylenediaminetetraacetic acid
- ELISA:
-
enzyme-linked immunosorbent assay
- FGF:
-
fibroblast growth factor
- FSH:
-
follicle-stimulating hormone
- GC:
-
granulosa cell
- H&E:
-
hematoxylin and eosin
- hAD-MSC:
-
human amnion–derived mesenchymal stem cell
- IGF:
-
insulin-like growth factor
- IL:
-
interleukin
- MSC:
-
mesenchymal stem cell
- OCT:
-
optimal cutting temperature
- POF:
-
premature ovarian failure
- POI:
-
primary ovarian insufficiency
- SD:
-
Sprague-Dawley
- VEGF:
-
vascular endothelial growth factor
- FBS:
-
fetal bovine serum
- E2:
-
estradiol
- ANOVA:
-
analysis of variance
- ERK:
-
extracellular signal–regulated kinase
- JNK:
-
c-Jun N-terminal kinase
- Akt:
-
protein kinase B
- PI3K:
-
phosphatidylinositol-3-kinase
- MKK:
-
MAPK kinase
- PF4:
-
platelet factor 4
- MAPK:
-
mitogen-activated protein kinase
- TNF:
-
tumor necrosis factor
- MMP:
-
matrix metalloproteinase
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–21.
Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28(32):4831–41.
Tonorezos ES, Hudson MM, Edgar AB, Kremer LC, Sklar CA, Wallace WH, et al. Screening and management of adverse endocrine outcomes in adult survivors of childhood and adolescent cancer. Lancet Diabetes Endocrinol. 2015;3(7):545–55.
Overbeek A, van den Berg MH, van Leeuwen FE, Kaspers GJ, Lambalk CB, van Dulmen-den Broeder E. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: a systematic review. Cancer Treat Rev. 2017;53:10–24.
Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–79.
Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82(3):222–9.
Tao XY, Zuo AZ, Wang JQ, Tao FB. Effect of primary ovarian insufficiency and early natural menopause on mortality: a meta-analysis. Climacteric. 2016;19(1):27–36.
Ling L, Feng X, Wei T, Wang Y, Wang Y, Zhang W, et al. Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats. Stem Cell Res Ther. 2017;8(1):283.
Luo Q, Yin N, Zhang L, Yuan W, Zhao W, Luan X, et al. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci. 2017;179:103–9.
Wei Wei, Ximei Wu, Li Y. Experimental methodology of pharmacology, 4th edition. 2010:1698.
Wang J, WU S, Shang H. Observation of estrous cycle in sexually mature female SD rats. Contemp Med. 2013;19(28):25–6.
Wang Z, Wang Y, Yang T, Li J, Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther. 2017;8(1):11.
Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17(3):555–7.
Faddy MJ. Follicle dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163(1–2):43–8.
European Society for Human R, Embryology Guideline Group on POI, Webber L, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–37.
Jensen AK, Rechnitzer C, Macklon KT, Ifversen MR, Birkebæk N, Clausen N, et al. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: focus on pubertal development. Hum Reprod. 2017;32(1):154–64.
Su J, Ding L, Cheng J, Yang J, Li X, Yan G, et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod. 2016;31(5):1075–86.
Levine JM, Kelvin JF, Quinn GP, Gracia CR. Infertility in reproductive-age female cancer survivors. Cancer. 2015;121(10):1532–9.
Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106(7):1588–99.
Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol. 2016;34(22):2568–74.
Herraiz S, Buigues A, Diaz-Garcia C, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109(5):908–18 e902.
Ling L, Feng X, Wei T, Wang Y, Wang Y, Wang Z, et al. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 2019;10(1):46.
Ling L, Wei T, He L, et al. Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif. 2017, 50(6):e12383.
Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561(7724):455–7.
Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–39.
Bukovsky A. Cell commitment by asymmetric division and immune system involvement. Prog Mol Subcell Biol. 2007;45:179–204.
Ye H, Zheng T, Li W, Li X, Fu X, Huang Y, et al. Ovarian stem cell nests in reproduction and ovarian aging. Cell Physiol Biochem. 2017;43(5):1917–25.
White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–21.
Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3(12):931–40.
Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany NY). 2009;1(12):971–8.
Massasa E, Costa XS, Taylor HS. Failure of the stem cell niche rather than loss of oocyte stem cells in the aging ovary. Aging (Albany NY). 2010;2(1):1–2.
Bukovsky A, Caudle MR. Immunoregulation of follicular renewal, selection, POF, and menopause in vivo, vs. neo-oogenesis in vitro, POF and ovarian infertility treatment, and a clinical trial. Reprod Biol Endocrinol. 2012;10:97.
Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V, et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26(7):1594–601.
Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27(6):1421–32.
Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155.
Zhang D, Liu Y, Zhang Z, Lv P, Liu Y, Li J, et al. Basonuclin 1 deficiency is a cause of primary ovarian insufficiency. Hum Mol Genet. 2018;27(21):3787–800.
Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018;25(3):261–9.
Rehnitz J, Alcoba DD, Brum IS, Hinderhofer K, Youness B, Strowitzki T, et al. FMR1 and AKT/mTOR signalling pathways: potential functional interactions controlling folliculogenesis in human granulosa cells. Reprod BioMed Online. 2017;35(5):485–93.
Yin N, Wang Y, Lu X, Liu R, Zhang L, Zhao W, et al. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res Ther. 2018;9(1):37.
Feng P, Li P, Tan J. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling. Stem Cell Rev. 2019, 15(2):241–255.
Kawamura K, Kawamura N, Hsueh AJ. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstet Gynecol. 2016;28(3):217–22.
Zhou Y, Qin Y, Qin Y, Xu B, Guo T, Ke H, et al. Wdr62 is involved in meiotic initiation via activating JNK signaling and associated with POI in humans. PLoS Genet. 2018;14(8):e1007463.
Kim J, Perez AS, Claflin J, David A, Zhou H, Shikanov A. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. NPJ Regen Med. 2016;1.16010.
Funding
This study was supported by the National Natural Science Foundation of China (81671415).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: XF and ZX; literature search: XF and LL; performed the experiments: XF, LL, WZ, XL, YL, and DT; analyzed the data: XF and LL; helped perform the analysis with constructive discussions: XF, LL, YW, and ZX; drafted the manuscript: XF and LL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This research was in accordance with the Helsinki Declaration and approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University.
Competing Interests
The authors declared that they have no conflict of interest.
Ethics Approval and Consent to Participate
The protocol was approved by the Committee on the Ethics of Animal Experiments of Chongqing Medical University (Permit Number: 2016-044).
Rights and permissions
About this article
Cite this article
Feng, X., Ling, L., Zhang, W. et al. Effects of Human Amnion–Derived Mesenchymal Stem Cell (hAD-MSC) Transplantation In Situ on Primary Ovarian Insufficiency in SD Rats. Reprod. Sci. 27, 1502–1512 (2020). https://doi.org/10.1007/s43032-020-00147-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00147-0