Abstract
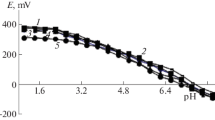
Complexation of iron(II) in aqueous glycine (α-aminoacetic acid) solutions is studied in the pH range 1.0–8.0 at 298.16 K at various ionic strengths (NaClO4). The stability constants of the resulting complexes go down as the ionic strength of the solution increases. The stability constants of the complexes are determined using the experimental “electromotive force of the system versus pH” curves by iterative fitting of the theoretical oxidation function to the experimental one with the Excel program. The correlation between the stability constants of complexes and ionic strength of the solution is also calculated using the Debye–Hückel equation and SigmaPlot 10.0 software. Statistical analysis of the calculated data is performed with P value of 0.95.
Similar content being viewed by others
References
Z. N. Yusupov, G. B. Eshova, and S. S. Saidov, Dokl. Akad. Nauk Resp. Tadzh. 51, 620 (2008).
L. V. Kvyatkovskaya, G. B. Eshova, D. A. Davlatshoeva, and M. M. Rakhimova, Vestn. TNU, Nos. 1–4 (153), 86 (2014).
The Chemist’s Handbook, Eds. by B. P. Nikol’skii and O. N. Grigorov (Khimiya, Moscow, 1965) [in Russian].
N. B. Berezin, A. G. Filippova, K. A. Sagdeev, and V. V. Chevela, Butlerov. Soobshch. 5, 39 (2004).
M. Beck and I. Nagepal, The Chemistry of Complex Equilibria (Akademiai Kiado, Budapest, 1988).
Yu. V. Koryakin and I. I. Angelov, Pure Chemical Substances (Khimiya, Moscow, 1974) [in Russian].
G. Charlot, Les methodes de la chimie analytique: Analyse quantitative minerale (Masson, Paris, 1961).
E. F. Faizulloev, M. A. Ismailova, Z. N. Yusupov, and M. M. Rakhimova, Vestn. TNU, No. 2(66), 33 (2011).
E. F. Faizulloev, M. M. Rakhimova, D. A. Davlatshoeva, et al., Vestn. TNU. Ser. Estestv. Nauk, No. 1/4(153), 66 (2014).
V. P. Vasil’ev, Thermodynamic Properties of Electrolyte Solutions (Vysshaya Shkola, Moscow, 1982) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.B. Eshova, J.A. Davlatshoeva, M.M. Rakhimova, M.O. Guriev, L.V. Kvyatkovskaya, 2018, published in Zhurnal Neorganicheskoi Khimii, 2018, Vol. 63, No. 6, pp. 736–740.
Rights and permissions
About this article
Cite this article
Eshova, G.B., Davlatshoeva, J.A., Rakhimova, M.M. et al. Formation of Glycinate Complexes of Iron(II) at Different Ionic Strengths of Solution. Russ. J. Inorg. Chem. 63, 772–776 (2018). https://doi.org/10.1134/S0036023618060098
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023618060098