Abstract
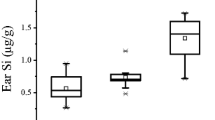
Both arginine and silicon affect collagen formation and bone mineralization. Thus, an experiment was designed to determine if dietary arginine would alter the effect of dietary silicon on bone mineralization and vice versa. Male weanling Sprague-Dawley rats were assigned to groups of 12 in a 2×2 factorially arranged experiment. Supplemented to a ground corn/casein basal diet containing 2.3 µg Si/g and adequate arginine were silicon as sodium metasilicate at 0 or 35 µg/g diet and arginine at 0 or 5 mg/g diet. The rats were fed ad libitum deionized water and their respective diets for 8 wk. Body weight, liver weight/body weight ratio, and plasma silicon were decreased, and plasma alkaline phosphatase activity was increased by silicon deprivation. Silicon deprivation also decreased femoral calcium, copper, potassium, and zinc concentrations, but increased the femoral manganese concentration. Arginine supplementation decreased femoral molybdenum concentration but increased the femoral manganese concentration. Vertebral concentrations of phosphorus, sodium, potassium, copper, manganese, and zinc were decreased by silicon deprivation. Arginine supplementation increased vertebral concentrations of sodium, potassium, manganese, zinc, and iron. The arginine effects were more marked in the silicon-deprived animals, especially in the vertebra. Germanium concentrations of the femur and vertebra were affected by an interaction between silicon and arginine; the concentrations were decreased by silicon deprivation in those animals not fed supplemental arginine. The change in germanium is consistent with a previous finding by us suggesting that this element may be physiologically important, especially as related to bone DNA concentrations. The femoral and vertebral mineral findings support the contention that silicon has a physiological role in bone formation and that arginine intake can affect that role.
Similar content being viewed by others
References
T. P. Kasten, P. Collin-Osdoby, N. Patel, P. Osdoby, M. Krukowski, T. P. Misko, et al., Potentiation of osteoblast bone-resporption activity by inhibition of nitric oxide synthase,Proc. Natl. Acad. Sci. USA 91, 3569–3573 (1994).
M. Fini, R. Giardino, N. Nicoli Aldini, L. Martini, M. Rocca, F. Bertoni, et al., Role of lactose, arginine and lysine combination in fracture healing,Ann. Ital. Chir. 67, 77–82 (1996) (in Italian).
E. M. Carlisle, Silicon, inHandbook of Nutritionally Essential Mineral Elements, B. L. O’Dell and R. A. Sunde, eds., Marcel Dekker, New York, pp. 603–618 (1997).
R. A. Wapnir, The absorption of arsenic, boron, silicon, aluminum, tin, iodine and fluorine: interactions with proteins and other nutrients, inProtein Nutrition and Mineral Absorption, R. A. Wapnir, ed., CRC, Boca Raton FL, pp. 277–308 (1990).
F. H. Nielsen and B. Bailey, The fabrication of plastic cages for suspension in mass air flow racks,Lab. Anim. Sci. 29, 502–506 (1979).
C. D. Seaborn and F. H. Nielsen, Effects of germanium and silicon on bone mineralization,Biol. Trace Element Res. 42, 151–164 (1994).
T. Gurney, Jr. and E. G. Gurney, DABA fluorescence assay for submicrogram amounts of DNA, inMethods in Molecular Biology, Vol. 2,Nucleic Acids, J. M. Walker, ed., Humana, Totowa, NJ, pp. 5–11 (1984).
F. E. Lichte, S. Hopper, and T. W. Osborn, Determination of silicon and aluminum in biological matrices by inductively coupled plasma emission spectrometry,Anal. Chem. 52, 120–124 (1980).
F. H. Nielsen, T. R. Shuler, T. J. Zimmerman, and E. O. Uthus, Magnesium and methionine deprivation affect the response of rats to boron deprivation,Biol. Trace Element Res. 17, 91–107 (1988).
SAS Institute, Inc.,SAS User’s Guide: Statistics Version, 5th ed., SAS Institute, Cary, NC (1985).
E. M. Carlisle, Silicon: an essential element for the chick,Science 178, 619–621 (1972).
K. Schwarz and D. B. Milne, Growth-promoting effects of silicon in rats,Nature 239, 333–334 (1972).
T. Tanaka, T. Nakanishi, Y. Hasuike, T. Inoue, K. Noguchi, and Y. Takamitsu, Paradoxical effect ofl-arginine on nitric oxide (NO) synthesis and histopathical changes in 5/6 nephrectomized SD rats,Nippon Jinzo Gakkai Shi 41, 754–63 (1999) (in Japanese).
E. M. Carlisle, Silicon,Nutr. Rev. 33, 257–261 (1975).
C. D. Seaborn and F. H. Nielsen, Dietary silicon effects acid and alkaline phosphatase and45calcium uptake in bone of rats,J. Trace Elements Exp. Med. 7, 11–18 (1994).
K. L. Watkins and L. L. Southern, Effect of dietary sodium zeolite A and graded levels of calcium on growth, plasma, and tibia characteristics of chicks,Poultry Sci. 70, 2295–2303 (1991).
R. M. Leach, Jr., B. S. Heinrichs, and J. Burdene, Broiler chicks fed low calcium diets. 1. Influence of zeolite on growth rate and parameters of bone metabolism,Poultry Sci. 69, 1539–1543 (1990).
J. Eisinger and D. Clairet, Effects of silicon, fluoride, etidronate and magnesium on bone mineral density: a retrospective study,Magnesium Res. 6, 247–249 (1993).
H. Rico, J. L. Gallego-Lago, E. R. Hernandez, L. F. Villa, A. Sanchez-Atrio, C. Seco, et al., Effect of silicon supplement on osteopenia induced by ovariectomy in rats,Calcif. Tissue Int. 66, 53–55 (2000).
M. Hott, C. de Pollak, D. Modrowski, and P. J. Marie, Short-term effects of organic silicon on trabecular bone in mature ovariectomized rats,Calcif. Tissue Int. 53, 174–179 (1993).
A. Schiano, F. Eisinger, P. Detolle, A. M. Laponche, B. Brisou, and J. Eisinger, Silicon, bone tissue and immunity (in French),Revue du Rhumatisme et des Maladies Osteo-Articulaires (Paris) 46, 483–486 (1979).
E. A. Ott and R. L. Asquith, Trace mineral supplementation of yearling horses,J. Anim. Sci. 73, 466–471 (1995).
E. M. Carlisle, A silicon requirement for normal skull formation,J. Nutr. 110, 352–359 (1980).
E. M. Carlisle, In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick,J. Nutr 106, 478–484 (1976).
N. Schutze, M. J. Oursler, J. Nolan, B. L. Riggs, and T. C. Spelsberg, Zeolite A inhibits osteoclast-mediated bone resorption in vitro,J. Cell. Biochem. 58, 39–46 (1995).
L. Connelly, M. Palacios-Callender, C. Ameixa, S. Moncada, and A. J. Hobbs, Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide,J. Immunol. 166, 3873–3881 (2001).
H. Tsukahara, M. Miura, S. Tsuchida, I. Hata, K. Hata, K. Yamamoto, et al., Effect of nitric oxide synthase inhibitors on bone metabolism in growing rats,Am. J. Physiol. 270, E840-E845 (1996).
Author information
Authors and Affiliations
Additional information
The U.S. Department of Agriculture, Agricultural Research Service, Northern Plains Area is an equal opportunity/affirmative action employer, and all agency services are available without discrimination.
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products that may be suitable.
Rights and permissions
About this article
Cite this article
Seaborn, C.D., Nielsen, F.H. Dietary silicon and arginine affect mineral element composition of rat femur and vertebra. Biol Trace Elem Res 89, 239–250 (2002). https://doi.org/10.1385/BTER:89:3:239
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:89:3:239