Abstract
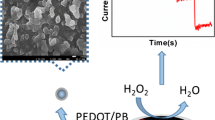
A novel non-enzymatic electrochemical sensor based on a nanoporous gold electrode modified with platinum nanoparticles was constructed for the determination of hydrogen peroxide (H2O2). Platinum nanoparticles exhibit good electrocatalytic activity towards hydrogen peroxide. The nanoporous gold (NPG) increases the effective surface area and has the capacity to promote electron-transfer reactions. With electrodeposition of Pt nanoparticles (NPs) on the surface of the nanoporous gold, the modified Au electrode afforded a fast, sensitive and selective electrochemical method for the determination of H2O2. The linear range for the detection of H2O2 was from 1.0 × 10−7 M to 2.0 × 10−5 M while the calculated limit of detection was 7.2 × 10−8 M on the basis of the 3σ/slope (σ represents the standard deviation of the blank samples). These findings could lead to the widespread use of electrochemical sensors to detect H2O2.
Similar content being viewed by others
References
Albero, B., Sánchez-Brunete, C., & Tadeo, J. L. (2003). Determination of organophosphorus pesticides in fruit juices by matrix solid-phase dispersion and gas chromatography. Journal of Agricultural and Food Chemistry, 51, 6915–6921. DOI: 10.1021/jf030414m.
Armstrong, F. A., & Wilson, G. S. (2000). Recent developments in faradaic bioelectrochemistry. Electrochimica Acta, 45, 2623–2645. DOI: 10.1016/s0013-4686(00)00342-x.
Bönnemann, H., & Richards, R. M. (2001). Nanoscopic metal particles — synthetic methods and potential applications. European Journal of Inorganic Chemistry, 10, 2455–2480. DOI: 10.1002/1099-0682(200109)2001:10〈2455::aid-ejic2455〉3.0.co;2-z.
Chen, S. H., Yuan, R., Chai, Y. Q., Yin, B., Li, W. J., & Min, L. G. (2009). Amperometric hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase on core-shell organosilica@chitosan nanospheres and multiwall carbon nanotubes composite. Electrochimica Acta, 54, 3039–3046. DOI: 10.1016/j.electacta.2008.12.009.
Clark, L. C. (1970). U.S. Patent No. 3539455. Washington, DC. USA: U.S. Patent and Trademark Office.
Gao, Z. Y., Liu, J. L., Chang, J. L., Wu, D. P., He, J. J., Wang, K., Xu, F., & Jiang, K. (2012). Mesocrystalline Cu2O hollow nanocubes: synthesis and application in non-enzymatic amperometric detection of hydrogen peroxide and glucose. CrystEngComm, 14, 6639–6646. DOI: 10.1039/c2ce25498k.
Heath, J. R. (1998). Covalency in semiconductor quantum dots. Chemical Society Reviews, 27, 65–71. DOI: 10.1039/a8270 65z.
Jia, J. B., Wang, B. Q., Wu, A. G., Cheng, G. J., Li, Z., & Dong, S. J. (2002). A method to construct a thirdgeneration horseradish peroxidase biosensor: Self-assembling gold nanoparticles to three-dimensional sol-gel network. Analytical Chemistry, 74, 2217–2223. DOI: 10.1021/ac011116w.
Kafi, A. K. M., Wu, G. S., & Chen, A. C. (2008). A novel hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase onto Au-modified titanium dioxide nanotube arrays. Biosensors and Bioelectronics, 24, 566–571. DOI: 10.1016/j.bios.2008.06.004.
Lases, E. C., Duurkens, V. A. M., Gerritsen, W. B. M., & Haas, F. J. L. M. (2000). Oxidative stress after lung resection therapy — A pilot study. Chest, 117, 999–1003. DOI: 10.1378/chest.117.4.999.
Lewis, L. N. (1993). Chemical catalysis by colloids and clusters. Chemical Reviews, 93, 2693–2730. DOI: 10.1021/cr00024a006.
Li, Z. Z., Cui, X. L., Zheng, J. S., Wang, Q. F., & Lin, Y. H. (2007). Effects of microstructure of carbon nanofibers for amperometric detection of hydrogen peroxide. Analytica Chimica Acta, 597, 238–244. DOI: 10.1016/j.aca.2007.06.046.
Lin, J. H., Zhang, L. J., & Zhang, S. S. (2007). Amperometric biosensor based on coentrapment of enzyme and mediator by gold nanoparticles on indium-tin oxide electrode. Analytical Biochemistry, 370, 180–185. DOI: 10.1016/j.ab.2007.06.021.
Link, S., & El-Sayed, M. A. (2003). Optical properties and ultrafast dynamics of metallic nanocrystals. Annual Review of Physical Chemistry, 54, 331–366. DOI: 10.1146/annurev. physchem.54.011002.103759.
Liu, G. D., & Lin, Y. H. (2005). Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Analytical Chemistry, 77, 5894–5901. DOI: 10.1021/ac050791t.
Lu, F. S., Gu, L. R., Meziani, M. J., Wang, X., Luo, P. G., Veca, L. M., Cao, L., & Sun, Y. P. (2009). Advances in bioapplications of carbon nanotubes. Advanced Materials, 21, 139–152. DOI: 10.1002/adma.200801491.
Ma, L. P., Yuan, R., Chai, Y. Q., & Chen, S. H. (2009). Amperometric hydrogen peroxide biosensor based on the immobilization of HRP on DNA-silver nanohybrids and PDDAprotected gold nanoparticles. Journal of Molecular Catalysis B: Enzymatic, 56, 215–220. DOI: 10.1016/j.molcatb.2008.05.007.
Mala Ekanayake, E. M. I., Preethichandra, D. M. G., & Kaneto, K. (2008). Bi-functional amperometric biosensor for low concentration hydrogen peroxide measurements using polypyrrole immobilizing matrix. Sensors and Actuators B: Chemical, 132, 166–171. DOI: 10.1016/j.snb.2008.01.042.
Niwa, O., Horiuchi, T., Kurita, R., & Torimitsu, K. (1998). On-line electrochemical sensor for selective continuous measurement of acetylcholine in cultured brain tissue. Analytical Chemistry, 70, 1126–1132. DOI: 10.1021/ac970257o.
Roucoux, A., Schulz, J., & Patin, H. (2002). Reduced transition metal colloids: A novel family of reusable catalysts? Chemical Reviews, 102, 3757–3778. DOI: 10.1021/cr010350j.
Roy, S., & Gao, Z. Q. (2009). Nanostructure-based electrical biosensors. Nano Today, 4, 318–334. DOI: 10.1016/j.nantod.2009.06.003.
Ruiz, B. L., Dempsey, E., Hua, C., Smyth, M. R., & Wang, J. (1993). Development of amperometric sensors for choline, acetylcholine and arsenocholine. Analytica Chimica Acta, 273, 425–430. DOI: 10.1016/0003-2670(93)80186-o.
Thenmozhi, K., & Narayanan, S. S. (2007). Amperometric hydrogen peroxide sensor based on a sol-gel-derived ceramic carbon composite electrode with toluidine blue covalently immobilized using 3-aminopropyltrimethoxysilane. Analytical and Bioanalytical Chemistry, 387, 1075–1082. DOI: 10.1007/s00216-006-0992-2.
Thomé-Duret, V., Reach, G., Gangnerau, M. N., Lemonnier, F., Klein, J. C., Zhang, Y. N., Hu, Y. B., & Wilson, G. S. (1996). Use of a subcutaneous glucose sensor to detect decreases in glucose concentration prior to observation in blood. Analytical Chemistry, 68, 3822–3826. DOI: 10.1021/ac960069i.
Vianello, F., Zennaro, L., & Rigo, A. (2007). A coulometric biosensor to determine hydrogen peroxide using a monomolecular layer of horseradish peroxidase immobilized on a glass surface. Biosensors and Bioelectronics, 22, 2694–2699. DOI: 10.1016/j.bios.2006.11.007.
Wang, H. S., Pan, Q. X., & Wang, G. X. (2005). A biosensor based on immobilization of horseradish peroxidase in chitosan matrix cross-linked with glyoxal for amperometric determination of hydrogen peroxide. Sensors, 5, 266–276. DOI: 10.3390/s5040266.
Xu, B., Ye, M. L., Yu, Y. X., & Zhang, W. D. (2010). A highly sensitive hydrogen peroxide amperometric sensor based on MnO2-modified vertically aligned multiwalled carbon nanotubes. Analytica Chimica Acta, 674, 20–26. DOI: 10.1016/j.aca.2010.06.004.
Xu, F. G., Sun, Y. J., Zhang, Y., Shi, Y., Wen, Z. W., & Li, Z. H. (2011). Graphene-Pt nanocomposite for nonenzymatic detection of hydrogen peroxide with enhanced sensitivity. Electrochemistry Communications, 13, 1131–1134. DOI: 10.1016/j.elecom.2011.07.017.
Yang, L., Janle, E., Huang, T. H., Gitzen, J., Kissinger, P. T., Vreeke, M., & Heller, A. (1995). Applications of “wired” peroxidase electrodes for peroxidase determination in liquid chromatography coupled to oxidase immobilized enzyme reactors. Analytical Chemistry, 67, 1326–1331 DOI: 10.1021/ac00104a005.
Yang, M. H., Yang, Y., Yang, H. F., Shen, G. L., & Yu, R. Q. (2006). Layer-by-layer self assembled multilayer films of carbon nanotubes and platinum nanoparticles with polyelectrolyte for the fabrication of biosensors. Biomaterials, 27, 246–255. DOI: 10.1016/j.biomaterials.2005.05.077.
Yorek, M. A. (2003). The role of oxidative stress in diabetic vascular and neural disease. Free Radical Research, 37, 471–480. DOI: 10.1080/1071576031000083161.
You, T. N., Niwa, O., Tomita, M., & Hirono, S. (2003). Characterization of platinum nanoparticle-embedded carbon film electrode and its detection of hydrogen peroxide. Analytical Chemistry, 75, 2080–2085. DOI: 10.1021/ac026337w.
Zhang, H. L., Lai, G. S., Han, D. Y., & Yu, A. M. (2008a). An amperometric hydrogen peroxide biosensor based on immobilization of horseradish peroxidase on an electrode modified with magnetic dextran microspheres. Analytical and Bioanalytical Chemistry, 390, 971–977. DOI: 10.1007/s00216-007-1748-3.
Zhang, T. T., Yuan, R., Chai, Y. Q., Li, W. J., & Ling, S. J. (2008b). A novel nonenzymatic hydrogen peroxide sensor based on a polypyrrole nanowire-copper nanocomposite modified gold electrode. Sensors, 8, 5141–5152. DOI: 10.3390/s8085141.
Zhou, G. Z., & Ju, H. X. (2004). Electrogenerated chemiluminescence from a CdSe nanocrystal film and its sensing application in aqueous solution. Analytical Chemistry, 76, 6871–6876. DOI: 10.1021/ac049012j.
Zhou, K. F., Zhu, Y. H., Yang, X. L., Luo, J., Li, C. Z., & Luan, S. R. (2010). A novel hydrogen peroxide biosensor based on Au-graphene-HRP-chitosan biocomposites. Electrochimica Acta, 55, 3055–3060. DOI: 10.1016/j.electacta.2010.01.035.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, G., Xing, L., Ma, XJ. et al. Non-enzymatic hydrogen peroxide sensor based on a nanoporous gold electrode modified with platinum nanoparticles. Chem. Pap. 68, 435–441 (2014). https://doi.org/10.2478/s11696-013-0473-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0473-y